Hsp22 Antibody (OASE00133)
OASE00133
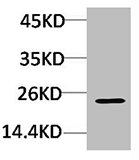
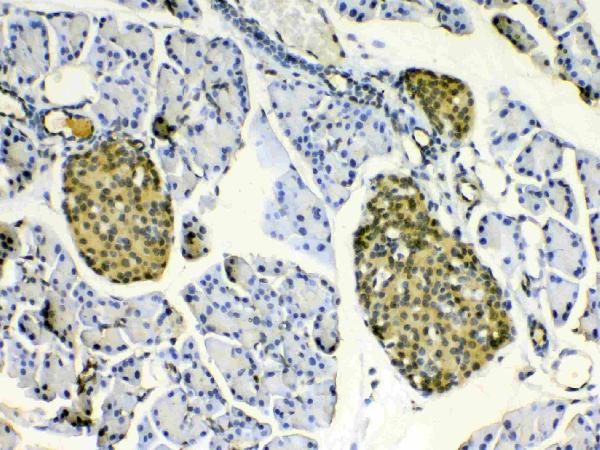
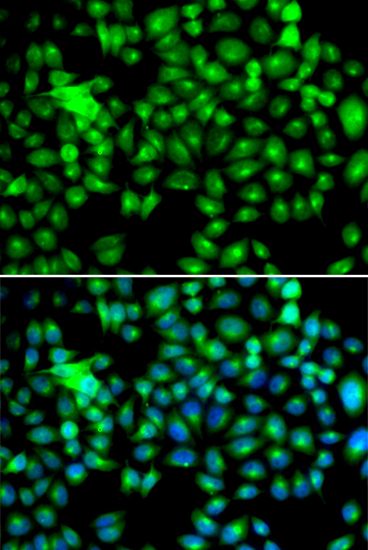
ApplicationsImmunoFluorescence, Western Blot, ELISA, ImmunoCytoChemistry, ImmunoHistoChemistry
Product group Antibodies
TargetHSPB8
Overview
- SupplierAviva Systems Biology
- Product NameHsp22 Antibody (OASE00133)
- Delivery Days Customer23
- ApplicationsImmunoFluorescence, Western Blot, ELISA, ImmunoCytoChemistry, ImmunoHistoChemistry
- CertificationResearch Use Only
- ClonalityMonoclonal
- Clone ID3C12-H11
- Concentration1 mg/ml
- ConjugateUnconjugated
- Gene ID26353
- Target nameHSPB8
- Target descriptionheat shock protein family B (small) member 8
- Target synonymsCMT2L, DHMN2, E2IG1, H11, HMN2, HMN2A, HMND2, HSP22, HSPB8-N1, HSPB8-N2, MFM13, heat shock protein beta-8, E2-induced gene 1 protein, alpha-crystallin C chain, heat shock 22kDa protein 8, heat shock 27kDa protein 8, heat shock protein family B member 8, protein kinase H11, small stress protein-like protein HSP22
- HostMouse
- IsotypeIgG1
- Scientific DescriptionHSP27s belong to an abundant and ubiquitous family of small heat shock proteins (sHSP). It is an important HSP found in both normal human cells and cancer cells. The basic structure of most sHSPs is a homologous and highly conserved amino acid sequence, with an I+/--crystallin domain at the C-terminus and the WD/EPF domain at the less conserved N-terminus. This N-terminus is essential for the development of high molecular oligomers (1, 2). HSP27-oligomers consist of stable dimers formed by as many as 8-40 HSP27 protein monomers (3). The oligomerization status is connected with the chaperone activity: aggregates of large oligomers have high chaperone activity, whereas dimers have no chaperone activity (4). HSP27 is localized to the cytoplasm of unstressed cells but can redistribute to the nucleus in response to stress, where it may function to stabilize DNA and/or the nuclear membrane. Other functions include chaperone activity (as mentioned above), thermo tolerance in vivo, inhibition of apoptosis, and signal transduction. Specifically, in vitro, it acts as an ATP independent chaperone by inhibiting protein aggregation and by stabilizing partially denatured proteins, which ensures refolding of the HSP70 complex. HSP27 is also involved in the apoptotic signaling pathway because it interferes with the activation of cytochrome c/Apaf-1/dATP complex, thereby inhibiting the activation of procaspase-9. It is also hypothesized that HSP27 may serve some role in cross-bridge formation between actin and myosin (5). And finally, HSP27 is also thought to be involved in the process of cell differentiation. The up-regulation of HSP27 correlates with the rate of phosphorylation and with an increase of large oligomers. It is possible that HSP27 may play a crucial role in termination of growth (6).
- Storage Instruction-20°C
- UNSPSC12352203