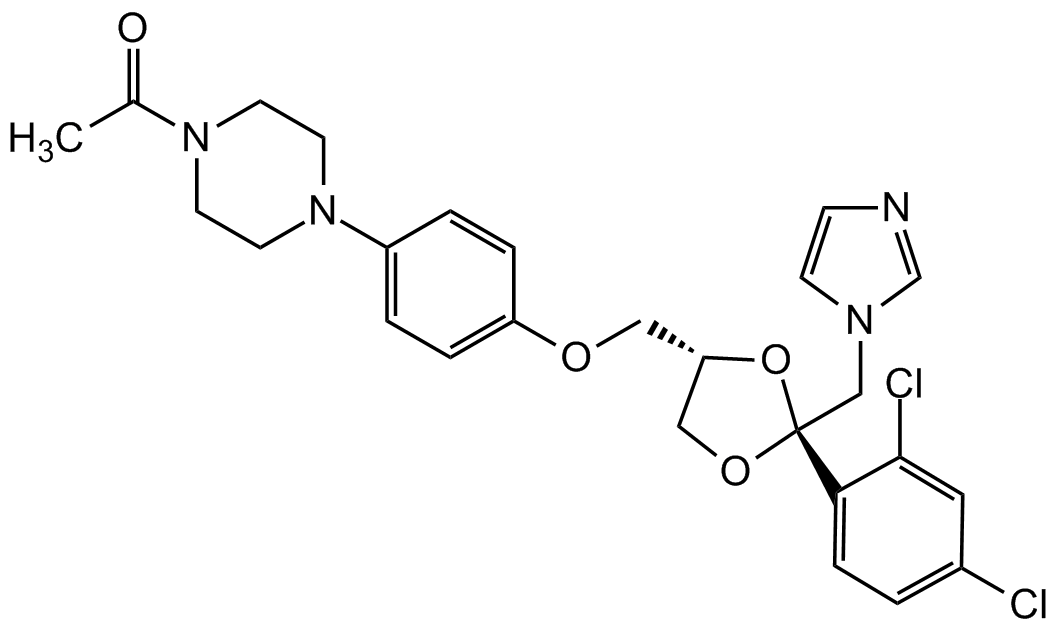
Chemical Structure
Ketoconazole [65277-42-1] [65277-42-1]
CDX-K0016
CAS Number65277-42-1
Product group Chemicals
Molecular Weight531.43
Overview
- SupplierChemodex
- Product NameKetoconazole [65277-42-1] [65277-42-1]
- Delivery Days Customer2
- CAS Number65277-42-1
- CertificationResearch Use Only
- Hazard InformationDanger,Excepted quantity
- Molecular FormulaC26H28Cl2N4O4
- Molecular Weight531.43
- Scientific DescriptionChemical. CAS: 65277-42-1. Formula: C26H28Cl2N4O4. MW: 531.43. Ketoconazole is a broad-spectrum triazole antifungal agent that has activity against C. albicans, C. krusei, C. tropicalis, C. glabrata, C. parapsilosis, C. neoformans and A. fumigatus strains (IC50s = 0.031-8 microg/ml). It inhibits the fungal cytochrome P450 (CYP) isoform CYP51, also known as lanosterol 14alpha-demethylase, which arrests ergosterol biosynthesis at the fungal membrane. This interaction inhibits ergosterol synthesis and results in increased fungal cellular permeability. Ketoconazole also inhibits human CYP3A4 (IC50 = 0.54 microM). Formulations containing ketoconazole have been used in the treatment of fungal infections. Ketoconazole blocks in humans activity of several enzymes necessary for the conversion of cholesterol to steroid hormones such as testosterone and cortisol. It has been shown to inhibit cholesterol side-chain cleavage enzyme, which converts cholesterol to pregnenolone, 17alpha-hydroxylase and 17,20-lyase, which convert pregnenolone into androgens, and 11beta-hydoxylase, which converts 11-deoxycortisol to cortisol. All of these enzymes are mitochondrial cytochrome p450 enzymes. Based on these antiandrogen and antiglucocorticoid effects, ketoconazole has been used with some success as a second-line treatment for certain forms of advanced prostate cancer and for the suppression of glucocorticoid synthesis in the treatment of Cushings syndrome. Ketoconazole displays potent anti-metastatic, anti-neoplastic, and anti-psoriatic activities and acts as an inhibitor of 5-lipoxygenase (5-LO) and thromboxane synthase activities. - Ketoconazole is a broad-spectrum triazole antifungal agent that has activity against C. albicans, C. krusei, C. tropicalis, C. glabrata, C. parapsilosis, C. neoformans and A. fumigatus strains (IC50s = 0.031-8 microg/ml). It inhibits the fungal cytochrome P450 (CYP) isoform CYP51, also known as lanosterol 14alpha-demethylase, which arrests ergosterol biosynthesis at the fungal membrane. This interaction inhibits ergosterol synthesis and results in increased fungal cellular permeability. Ketoconazole also inhibits human CYP3A4 (IC50 = 0.54 microM). Formulations containing ketoconazole have been used in the treatment of fungal infections. Ketoconazole blocks in humans activity of several enzymes necessary for the conversion of cholesterol to steroid hormones such as testosterone and cortisol. It has been shown to inhibit cholesterol side-chain cleavage enzyme, which converts cholesterol to pregnenolone, 17alpha-hydroxylase and 17,20-lyase, which convert pregnenolone into androgens, and 11beta-hydoxylase, which converts 11-deoxycortisol to cortisol. All of these enzymes are mitochondrial cytochrome p450 enzymes. Based on these antiandrogen and antiglucocorticoid effects, ketoconazole has been used with some success as a second-line treatment for certain forms of advanced prostate cancer and for the suppression of glucocorticoid synthesis in the treatment of Cushings syndrome. Ketoconazole displays potent anti-metastatic, anti-neoplastic, and anti-psoriatic activities and acts as an inhibitor of 5-lipoxygenase (5-LO) and thromboxane synthase activities.
- SMILESCC(N(CC1)CCN1C2=CC=C(OC[C@H]3CO[C@](CN4C=NC=C4)(C5=CC=C(Cl)C=C5Cl)O3)C=C2)=O
- Storage Instruction-20°C,2°C to 8°C
- UN Number2811
- UNSPSC12352200


![Ketoconazole [65277-42-1] [65277-42-1]](https://www.targetmol.com/group3/M00/03/0B/CgoaEGY7RTCETO4IAAAAAAm-H1E026.png)