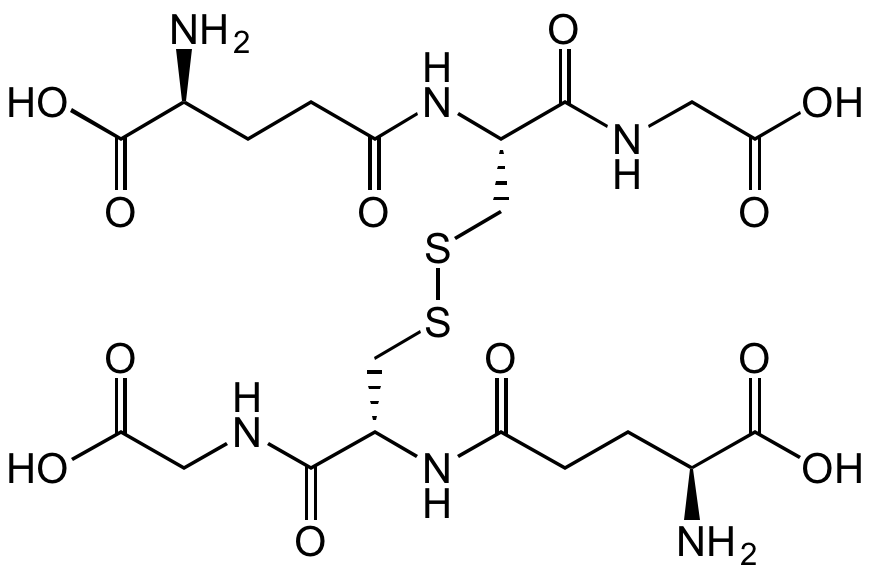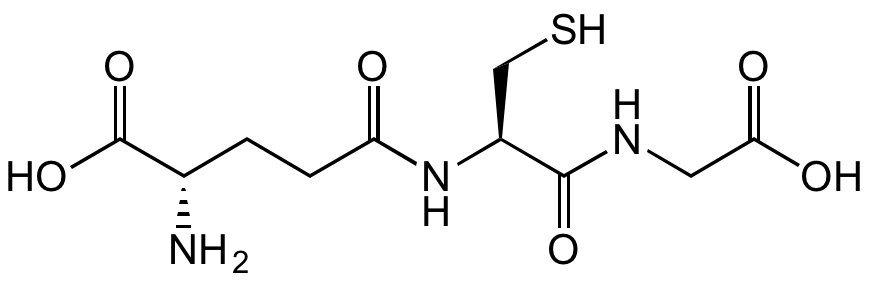
Chemical Structure
L-Glutathione reduced [70-18-8] [70-18-8]
CDX-G0005
CAS Number70-18-8
Product group Chemicals
Estimated Purity>98%
Molecular Weight307.32
Overview
- SupplierChemodex
- Product NameL-Glutathione reduced [70-18-8] [70-18-8]
- Delivery Days Customer2
- CAS Number70-18-8
- CertificationResearch Use Only
- Estimated Purity>98%
- Molecular FormulaC10H17N3O6S
- Molecular Weight307.32
- Scientific DescriptionChemical. CAS: 70-18-8. Formula: C10H17N3O6S. MW: 307.32. Synthetic. Major tripeptide widely distributed in both plants and animals. Endogenous antioxidant that plays a major role in reducing reactive oxygen species formed during cellular metabolism and the respiratory burst. Regulates activity of the redox sensitive transcription factor NF-kappaB. Cytoprotective. Serves as a nucleophilic co-substrate to glutathione transferases in the detoxification of xenobiotics and is an essential electron donor to glutathione peroxidases in the reduction of hydroperoxides. Involved in amino acid transport and maintenance of protein sulfhydryl reduction status. Posseses several metabolic, regulatory and protective functions. Forms disulfide bonds with cysteine residues in proteins. - Major tripeptide widely distributed in both plants and animals. Endogenous antioxidant that plays a major role in reducing reactive oxygen species formed during cellular metabolism and the respiratory burst. Regulates activity of the redox sensitive transcription factor NF-kappaB. Cytoprotective. Serves as a nucleophilic co-substrate to glutathione transferases in the detoxification of xenobiotics and is an essential electron donor to glutathione peroxidases in the reduction of hydroperoxides. Involved in amino acid transport and maintenance of protein sulfhydryl reduction status. Posseses several metabolic, regulatory and protective functions. Forms disulfide bonds with cysteine residues in proteins.
- SMILESOC([C@H](CCC(N[C@@H](CS)C(NCC(O)=O)=O)=O)N)=O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200


![L-Glutathione reduced [70-18-8]](https://www.targetmol.com/group3/M00/03/00/CgoaEWY7PsyEUUzmAAAAACtXkpw401.png)