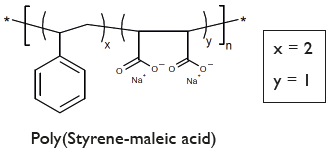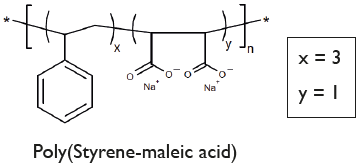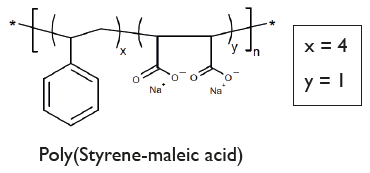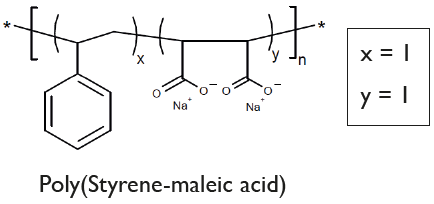
Chemical Structure
SMA-100 [Lipodisq(tm) Styrene:Maleic Acid Copolymer 1:1]
IAX-700-201
Estimated Purity>98%
Product group Chemicals
Molecular Weight5,500 (based on weight)2,100 (based on number)
Overview
- SupplierInnaxon
- Product NameSMA-100 [Lipodisq(tm) Styrene:Maleic Acid Copolymer 1:1]
- Delivery Days Customer10
- CertificationResearch Use Only
- Estimated Purity>98%
- Molecular FormulaC12H10O4Na2
- Molecular Weight5,500 (based on weight)2,100 (based on number)
- Scientific DescriptionA nanoparticle (11-40nm) drug delivery system comprising a discoidal phospholipid bilayer membrane stabilized by a chaperone molecular annulus. Lipodisq™ polymers are available as 4 defined structures (1:1, 2:1, 3:1, and 4:1 Styrene to MaleicAcid (SMA) rations) each individually operational within a selected pH range for optimal working conditions. Components are batch-tested for Lipodisq™ formation using buffer systems available, which are tested for nano-formulated drug analysis by Dynamic Light Scattering (DLS). These buffer solutions are endotoxin-tested and sterile. Lipodisq™ formation is highly efficient and nanodiscs show a good safety profile and are suitable for in vitro and in vivo (in experimental animals) investigations. Lipodisq™ are nanosized lipid-based discoidal particles to incorporate hydrophobic, poorly water-soluble active compounds, such as peptides, lipids, lipoproteins, transmembrane proteins and glycolipids. Applications of Lipodisq™ include functional and structural characterization of the cargo and drug delivery with improved bioavailability, and biological half-life in vivo (PD/PK) or delivery of antigens preserved in their native conformation for immunization purposes. Answering the call for Membrane Protein (MP) and MP reconstituting membrane simulants which do not necessitate the use of mediating detergent, is a copolymer of styrene and maleic anhydride subsequently hydrolyzed into amphipathic polystyrene-co-maleic acid (SMA). Nanodiscs resulting from SMA are also referred to as styrene-maleic acid-lipid particles (SMALP). SMA-lipid particles are formed by directly extracting membrane proteins either from native cellular membranes (giving native nanodiscs) or from an intermediary MP-reconstituted synthetic membrane system to ultimately form self-assembled nanodisc structures of a general 10-12 nm diameter. The demonstration of SMA-lipid particles as a monodisperse MP reconstitution system was reported in 2009, although the interaction of SMA with phospholipids to generate disc-shaped structures (now known as empty lipid nanodiscs), was previously established and investigated for use as a drug delivery system years preceding this discovery. At a physiological pH of 7-8, SMA monomer ratios of 2:1 and 3:1 are most commonly used due to their optimal efficiency for nanodisc extraction from phospholipid bilayers. The ability of SMA to create monodisperse nanodiscs with size flexibility facilitates the reconstitution of a range of oligomeric MPs and MP complexes for analysis by various studies including fluorescence microscopy, NMR, and single-particle cryo-EM. Whereas in a statistical version of SMA, like the commercial Malvern polymer LipodisqTM, monomers are evenly distributed throughout the polymer chain sequence in proportion to their ratio as well as exhibiting greater dispersity in chain length. Published procedures using a ratio of 2:1 (w/w) for P(SMA)/SMA polymer and phospholipid, such as 100mg P(SMA)/SMA polymer and 50mg DMPC. Selected hydrophobic drug candidates or peptides or transmembrane proteins (MP) are mixed with the aqueous phospholipid emulsionmaking up 2.5%. This is stirred at temperatures above the phase transition temperature of the lipid (>24°C for DMPC) before aqueous P(SMA)/SMA polymer at 5% is added drop-wise and with pauses until an approximate volume ratio of 1:1 is reached and the lipid emulsion clears. Alternatively, P(SMA)/SMA polymer solutions are mixed with native (cell or bacterial) membranes to form native nanodiscs. Stirring time, pH, selected buffer type and strength (e.g. HEPES, NaCl, TRIS or PBS w/o Ca++ and Mg++) and temperature of the phospholipid emulsion containing the MP or active ingredient, need to be optimized. Further purification of formed LipodisqTM can be achieved by ultracentrifugation at >100,000 x g to remove residual lipid, surplus P(SMA)polymer with the Lipodisq™ nanodiscs remaining in the supernatant. Alternatively, size exclusion (SEC) methods can also be applied. - Chemical. CAS: 26762-29-8. Formula: C12H10O4Na2. MW: 5,500 (based on weight)2,100 (based on number). Soluble in water, and buffer solutions (pH 3.50-5.50) to allow the formulation of a proprietary, thermostable, aqueous lipid nanoparticle. A nanoparticle (11-40nm) drug delivery system comprising a discoidal phospholipid bilayer membrane stabilized by a chaperone molecular annulus. Lipodisq™ polymers are available as 4 defined structures (1:1, 2:1, 3:1, and 4:1 Styrene to MaleicAcid (SMA) rations) each individually operational within a selected pH range for optimal working conditions. Components are batch-tested for Lipodisq™ formation using buffer systems available, which are tested for nano-formulated drug analysis by Dynamic Light Scattering (DLS). These buffer solutions are endotoxin-tested and sterile. Lipodisq™ formation is highly efficient and nanodiscs show a good safety profile and are suitable for in vitro and in vivo (in experimental animals) investigations. Lipodisq™ are nanosized lipid-based discoidal particles to incorporate hydrophobic, poorly water-soluble active compounds, such as peptides, lipids, lipoproteins, transmembrane proteins and glycolipids. Applications of Lipodisq™ include functional and structural characterization of the cargo and drug delivery with improved bioavailability, and biological half-life in vivo (PD/PK) or delivery of antigens preserved in their native conformation for immunization purposes. Answering the call for Membrane Protein (MP) and MP reconstituting membrane simulants which do not necessitate the use of mediating detergent, is a copolymer of styrene and maleic anhydride subsequently hydrolyzed into amphipathic polystyrene-co-maleic acid (SMA). Nanodiscs resulting from SMA are also referred to as styrene-maleic acid-lipid particles (SMALP). SMA-lipid particles are formed by directly extracting membrane proteins either from native cellular membranes (giving native nanodiscs) or from an intermediary MP-reconstituted synthetic membrane system to ultimately form self-assembled nanodisc structures of a general 10-12 nm diameter. The demonstration of SMA-lipid particles as a monodisperse MP reconstitution system was reported in 2009, although the interaction of SMA with phospholipids to generate disc-shaped structures (now known as empty lipid nanodiscs), was previously established and investigated for use as a drug delivery system years preceding this discovery. At a physiological pH of 7-8, SMA monomer ratios of 2:1 and 3:1 are most commonly used due to their optimal efficiency for nanodisc extraction from phospholipid bilayers. The ability of SMA to create monodisperse nanodiscs with size flexibility facilitates the reconstitution of a range of oligomeric MPs and MP complexes for analysis by various studies including fluorescence microscopy, NMR, and single-particle cryo-EM. Whereas in a statistical version of SMA, like the commercial Malvern polymer LipodisqTM, monomers are evenly distributed throughout the polymer chain sequence in proportion to their ratio as well as exhibiting greater dispersity in chain length. Published procedures using a ratio of 2:1 (w/w) for P(SMA)/SMA polymer and phospholipid, such as 100mg P(SMA)/SMA polymer and 50mg DMPC. Selected hydrophobic drug candidates or peptides or transmembrane proteins (MP) are mixed with the aqueous phospholipid emulsionmaking up 2.5%. This is stirred at temperatures above the phase transition temperature of the lipid (>24°C for DMPC) before aqueous P(SMA)/SMA polymer at 5% is added drop-wise and with pauses until an approximate volume ratio of 1:1 is reached and the lipid emulsion clears. Alternatively, P(SMA)/SMA polymer solutions are mixed with native (cell or bacterial) membranes to form native nanodiscs. Stirring time, pH, selected buffer type and strength (e.g. HEPES, NaCl, TRIS or PBS w/o Ca++ and Mg++) and temperature of the phospholipid emulsion containing the MP or active ingredient, need to be optimized. Further purification of formed LipodisqTM can be achieved by ultracentrifugation at >100,000 x g to remove residual lipid, surplus P(SMA)polymer with the Lipodisq™ nanodiscs remaining in the supernatant. Alternatively, size exclusion (SEC) methods can also be applied.
- Storage Instruction2°C to 8°C
- UNSPSC12352200