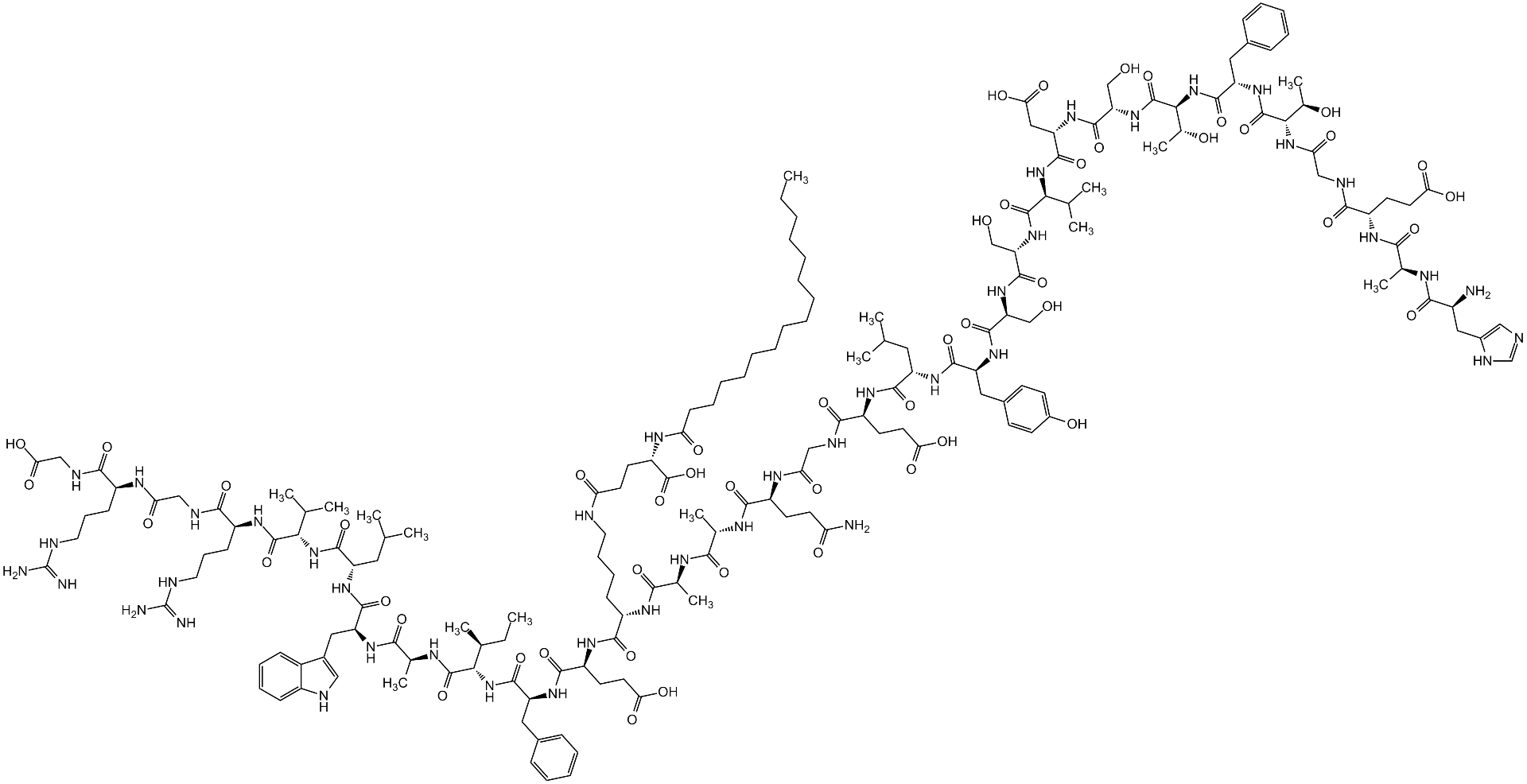
Chemical Structure
Liraglutide [204656-20-2] [204656-20-2]
AG-CP3-0034
CAS Number204656-20-2
Product group Chemicals
Estimated Purity>98%
Molecular Weight3751.2
Overview
- SupplierAdipoGen Life Sciences
- Product NameLiraglutide [204656-20-2] [204656-20-2]
- Delivery Days Customer10
- CAS Number204656-20-2
- CertificationResearch Use Only
- Estimated Purity>98%
- Molecular FormulaC172H265N43O51
- Molecular Weight3751.2
- Scientific DescriptionChemical. CAS: 204656-20-2. Formula: C172H265N43O51. MW: 3751.2. Synthetic. Long-acting acylated glucagon-like peptide-1 (GLP-1) receptor agonist. Antidiabetic and antiobesity agent used clinically to treat type 2 diabetes mellitus. Binding to GLP-1R, activates AMP-activated protein kinase, consequently stimulates insulin secretion in pancreatic beta cells and suppresses glucagon secretion in a glucose-dependent manner. Improves control of blood glucose and consequently modulates appetite and body weight. Inhibits beta cell apoptosis and improves beta cell mass. Shown to ameliorate glycometabolism and insulin resistance through the upregulation of GLUT4. Neuroprotective. Prevents neurodegenerative processes in pathologies such as Alzheimers and Parkinsons Disease. Anti-inflammatory agent. Exerts cardioprotective roles via activating prosurvival pathways and suppressing inflammation. Anti-pyroptotic by inhibiting TNF-alpha and hypoxia-induced inflammasome activation. Anticancer agent. Inhibited proliferation and induced apoptosis in cancer cell lines. Shown to inhibit osteoclastogenesis. - Long-acting acylated glucagon-like peptide-1 (GLP-1) receptor agonist. Antidiabetic and antiobesity agent used clinically to treat type 2 diabetes mellitus. Binding to GLP-1R, activates AMP-activated protein kinase, consequently stimulates insulin secretion in pancreatic beta cells and suppresses glucagon secretion in a glucose-dependent manner. Improves control of blood glucose and consequently modulates appetite and body weight. Inhibits beta cell apoptosis and improves beta cell mass. Shown to ameliorate glycometabolism and insulin resistance through the upregulation of GLUT4. Neuroprotective. Prevents neurodegenerative processes in pathologies such as Alzheimers and Parkinsons Disease. Anti-inflammatory agent. Exerts cardioprotective roles via activating prosurvival pathways and suppressing inflammation. Anti-pyroptotic by inhibiting TNF-alpha and hypoxia-induced inflammasome activation. Anticancer agent. Inhibited proliferation and induced apoptosis in cancer cell lines. Shown to inhibit osteoclastogenesis.
- SMILESO=C([C@H](CC(C)C)N([H])C([C@H](CC1=CN([H])C2=CC=CC=C12)N([H])C([C@H](C)N([H])C([C@H]([C@@H](C)CC)N([H])C([C@H](CC3=CC=CC=C3)N([H])C([C@H](CCC(O)=O)N([H])C([C@H](CCCCN([H])C(CC[C@@H](C(O)=O)N([H])C(CCCCCCCCCCCCCCC)=O)=O)N([H])C([C@H](C)N([H])C([C@H](C)N([H])C([C@H](CCC(N([H])[H])=O)N([H])C(CN([H])C([C@H](CCC(O)=O)N([H])C([C@H](CC(C)C)N([H])C([C@H](CC(C=C4)=CC=C4O)N([H])C([C@H](CO)N([H])C([C@H](CO)N([H])C([C@H](C(C)C)N([H])C([C@H](CC(O)=O)N([H])C([C@H](CO)N([H])C([C@H]([C@@H](C)O)N([H])C([C@H](CC5=CC=CC=C5)N([H])C([C@H]([C@@H](C)O)N([H])C(CN([H])C([C@H](CCC(O)=O)N([H])C([C@H](C)N([H])C([C@H](CC6=CN=CN6[H])N([H])[H])=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)N([H])[C@H](C(N([H])[C@H](C(N([H])CC(N([H])[C@H](C(N([H])CC(O)=O)=O)CCCN([H])/C(N([H])[H])=N/[H])=O)=O)CCCN([H])/C(N([H])[H])=N/[H])=O)C(C)C
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200


![Liraglutide [204656-20-2]](https://www.targetmol.com/group3/M00/03/0A/CgoaEWY7P_mEeVxbAAAAAIZ-zFg151.png)