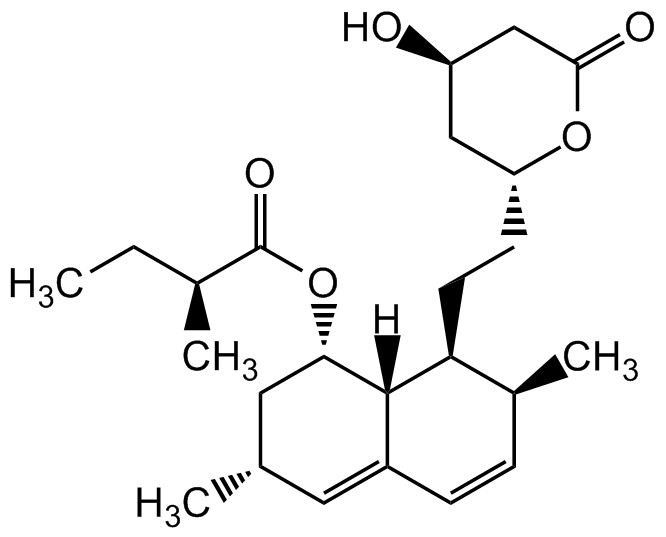
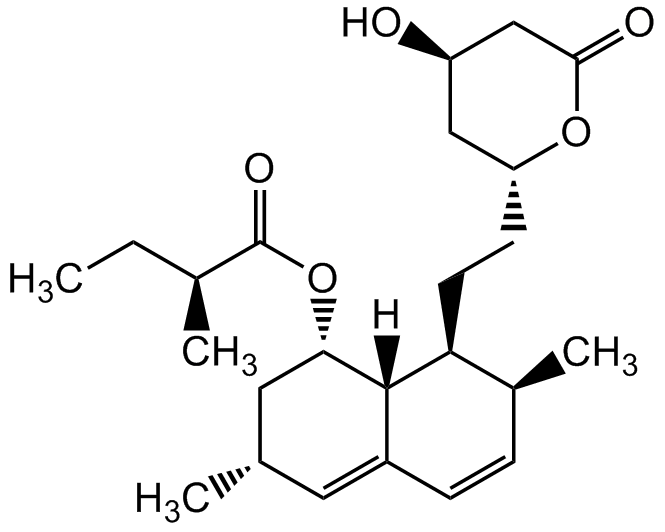
Chemical Structure
Lovastatin
AG-CN2-0051
CAS Number75330-75-5
Product group Chemicals
Estimated Purity>98%
Molecular Weight404.6
Overview
- SupplierAdipoGen Life Sciences
- Product NameLovastatin
- Delivery Days Customer10
- CAS Number75330-75-5
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationWarning
- Molecular FormulaC24H36O5
- Molecular Weight404.6
- Scientific DescriptionChemical. CAS: 75330-75-5. Formula: C24H36O5. MW: 404.6. Synthetic. Statin compound. Potent competitive HMG-CoA reductase inhibitor. Anti-hypercholesterolemic agent. Cholesterol/isoprenoid biosynthesis inhibitor. Blocks the production of mevalonate, a critical compound in the production of cholesterol and isoprenoids. Inhibits the isoprenylation of Ras and Rho family GTPases. Causes cell cycle arrest in G1 and G2/M phases through modulation of proteasome in cancer cell lines. Smooth muscle cell proliferation inhibitor. Apoptosis inducer. Anticancer compound. Anti-adhesive, immunomodulatory and anti-inflammatory compound. Stimulates bone formation. Increases cellular resistance to anticancer agents such as doxorubicin and etoposide (Prod. No. AG-CR1-3572 http://www.adipogen.com/ag-cr1-3572/etoposide.html ). Suppresses ICAM-1-LFA-1 interactions, which blocks virus replication and infection. Anti-hypertensive agent. Suppresses TNF-induced NF-kappaB activation. Modulates key cell signaling pathways, like Ras, MAPK and EGFR (modest). - Statin compound. Potent competitive HMG-CoA reductase inhibitor. Anti-hypercholesterolemic agent. Cholesterol/isoprenoid biosynthesis inhibitor. Blocks the production of mevalonate, a critical compound in the production of cholesterol and isoprenoids. Inhibits the isoprenylation of Ras and Rho family GTPases . Anticancer compound that causes cell cycle arrest in G1 and G2/M phases through modulation of proteasome in cancer cell lines. SKP2 E3 ligase inhibitor. Decreases the expression of Skp2 and results in the inhibition of Skp2-mediated ubiquitination and degradation of p27 and p21, leading to cell cycle arrest and apoptosis. Smooth muscle cell proliferation inhibitor. Anti-adhesive, immunomodulatory and anti-inflammatory compound that stimulates bone formation. Suppresses TNF-induced NF-kappaB activation. Increases cellular resistance to anticancer agents such as doxorubicin and etoposide (Prod. No. AG-CR1-3572 http://www.adipogen.com/ag-cr1-3572/etoposide.html ). Suppresses ICAM-1-LFA-1 interactions, which blocks virus replication and infection. Anti-hypertensive agent. Modulates key cell signaling pathways, like Ras, MAPK and EGFR (modest). Lovastatin-treated neurons exhibit shortened neurite outgrowth.
- SMILES[H][C@]12[C@H](C[C@@H](C)C=C1C=C[C@H](C)[C@@H]2CC[C@@H]1C[C@@H](O)CC(=O)O1)OC(=O)[C@@H](C)CC
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200

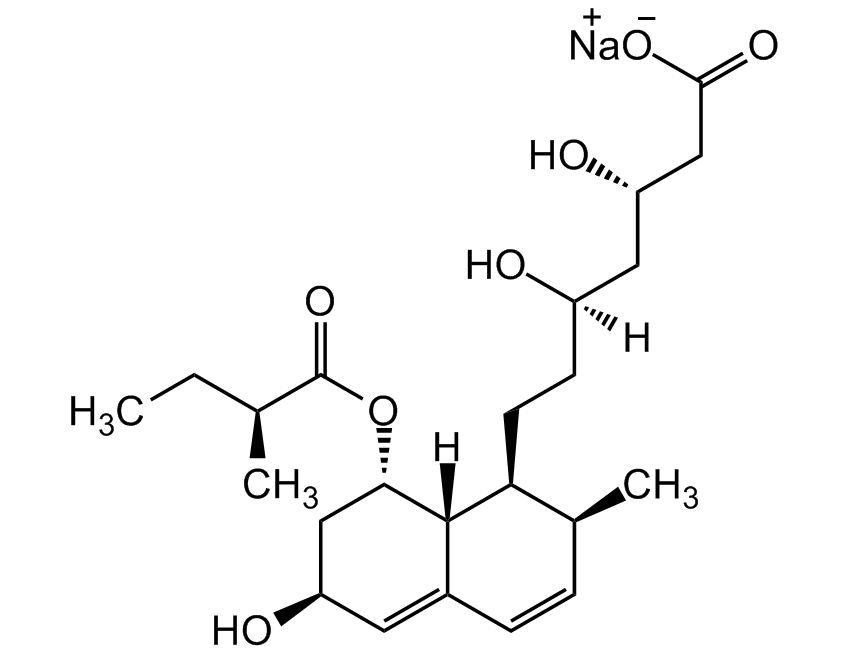
![Lovastatin [75330-75-5]](https://www.targetmol.com/group3/M00/34/7E/CgoaEGarftyEc3t8AAAAAIrPDUM182.png)
