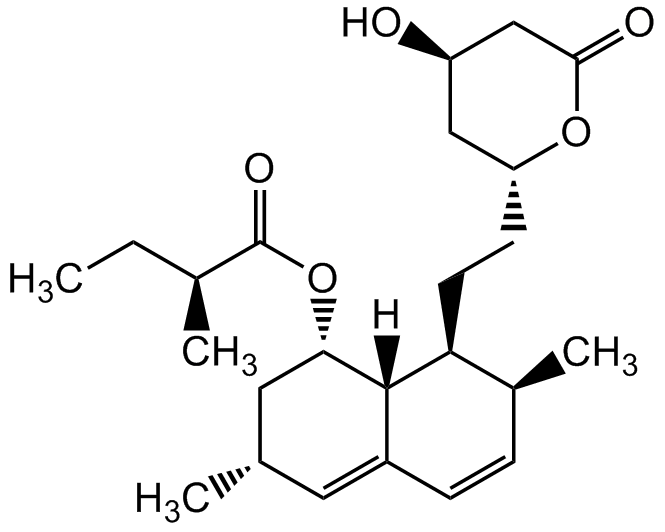
Chemical Structure
Lovastatin [75330-75-5] [75330-75-5]
CDX-L0281
CAS Number75330-75-5
Product group Chemicals
Estimated Purity>98%
Molecular Weight404.54
Overview
- SupplierChemodex
- Product NameLovastatin [75330-75-5] [75330-75-5]
- Delivery Days Customer2
- CAS Number75330-75-5
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationWarning
- Molecular FormulaC24H36O5
- Molecular Weight404.54
- Scientific DescriptionChemical. CAS: 75330-75-5. Formula: C24H36O5. MW: 404.54. Lovastatin is an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA reductase), an enzyme that catalyzes the conversion of HMG-CoA to mevalonate. Lovastatin is a prodrug, which is hydrolyzed in vivo to the active beta-hydroxy acid open ring form. Mevalonate is a required building block for cholesterol biosynthesis and lovastatin interferes with its production by acting as a reversible competitive inhibitor for HMG-CoA. Lovastatin is an effective anti-hypercholesterolemic agent widely used as a lipid-lowering drug. In addition to lowering blood lipid levels, Lovastatin also has been shown to have anticancer, neuroprotective, anti-inflammatory, antiviral and antibacterial properties. - Lovastatin is an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA reductase), an enzyme that catalyzes the conversion of HMG-CoA to mevalonate. Lovastatin is a prodrug, which is hydrolyzed in vivo to the active beta-hydroxy acid open ring form. Mevalonate is a required building block for cholesterol biosynthesis and lovastatin interferes with its production by acting as a reversible competitive inhibitor for HMG-CoA. Lovastatin is an effective anti-hypercholesterolemic agent widely used as a lipid-lowering drug. In addition to lowering blood lipid levels, Lovastatin also has been shown to have anticancer, neuroprotective, anti-inflammatory, antiviral and antibacterial properties.
- SMILESC[C@H]1C=CC2=C[C@H](C)C[C@H](OC([C@@H](C)CC)=O)[C@]2([H])[C@H]1CC[C@H]3OC(C[C@H](O)C3)=O
- Storage Instruction2°C to 8°C,RT
- UNSPSC12352200


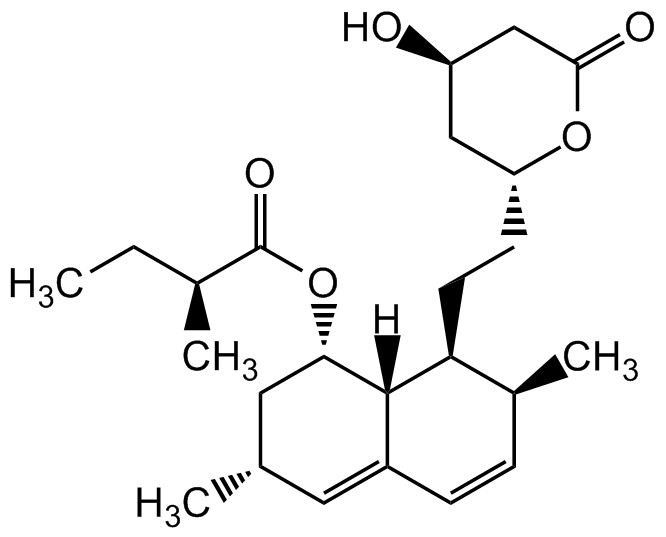
![Lovastatin [75330-75-5] [75330-75-5]](https://www.targetmol.com/group3/M00/34/7E/CgoaEGarftyEc3t8AAAAAIrPDUM182.png)