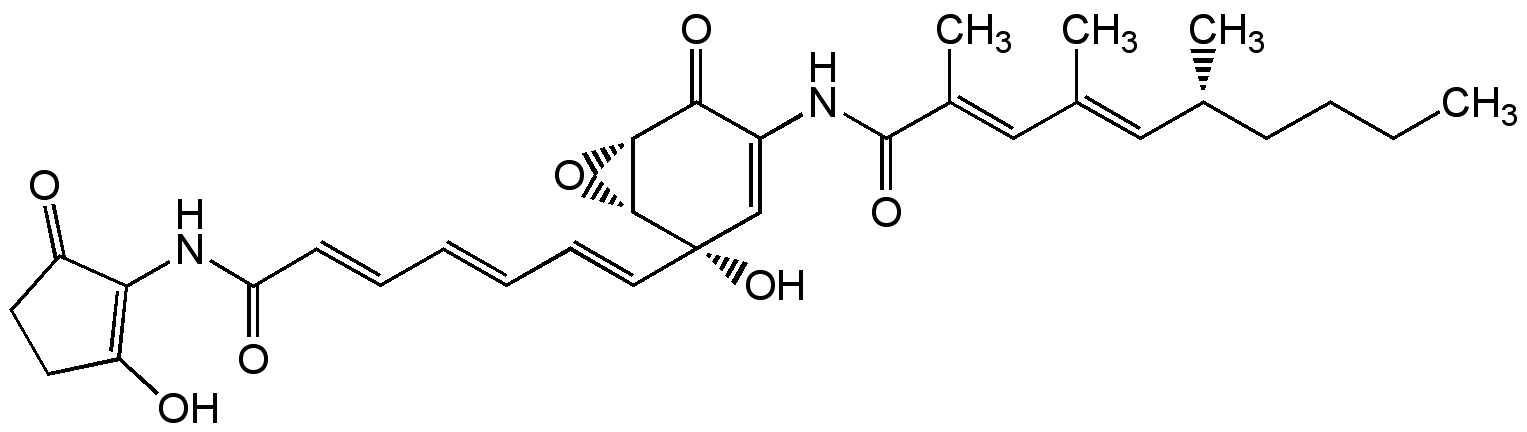
Chemical Structure
Manumycin A [52665-74-4]
BVT-0091
CAS Number52665-74-4
Product group Chemicals
Estimated Purity>98%
Molecular Weight550.6
Overview
- SupplierBioViotica
- Product NameManumycin A [52665-74-4]
- Delivery Days Customer10
- CAS Number52665-74-4
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationWarning
- Molecular FormulaC31H38N2O7
- Molecular Weight550.6
- Scientific DescriptionAntibiotic. Apoptosis and endoplasmic reticulum stress-mediated cell death inducer. Potent, selective and competitive cell permeable rasfarnesyltransferase inhibitor (IC50 = 30 nM). Does not affect geranylgeranyltransferase (IC50 = 180 microM). Inhibition is competitive with respect to farnesyl pyrophosphate and non-competitive with respect to Ras. Neutral sphingomyelinase inhibitor. Blocks insulin-induced MAP kinase activation in rat cardiac myocytes (19 microM). Targets protein phosphatase 1alpha (PP1alpha) and reduces hydrogen peroxide. Corrects aberrant splicing of the muscle chloride channel Clcn1 in myotonic dystrophy type 1 (DM1). Shows potential anti-inflammatory activity. Irreversible human thioredoxin reductase 1 (TrxR-1) inhibitor (IC50=272nM) and inducer of NADPH oxidase activity. Acts very likely as a Michael acceptor to the nucleophilic Sec residue in the C-terminal redox center of TrxR-1 which yields a SecTRAP (selenium compromised thioredoxin reductase-derived apoptotic proteins), which promotes both apoptosis and necrosis via oxidative stress and increased intracellular reactive oxygen species (ROS) production. Due to the involvement of Ras during exosomes release, manumycin A has been investigated as an inhibitor of exosomes secretion. Acts against myotonic dystrophy type 1 (DM1). Mediates lymphoma apoptosis by targeting protein phosphatase 1alpha. - Chemical. CAS: 52665-74-4. Formula: C31H38N2O7. MW: 550.6. Isolated from Streptomyces parvulus. Antibiotic. Apoptosis and endoplasmic reticulum stress-mediated cell death inducer. Potent, selective and competitive cell permeable rasfarnesyltransferase inhibitor (IC50 = 30 nM). Does not affect geranylgeranyltransferase (IC50 = 180 microM). Inhibition is competitive with respect to farnesyl pyrophosphate and non-competitive with respect to Ras. Neutral sphingomyelinase inhibitor. Blocks insulin-induced MAP kinase activation in rat cardiac myocytes (19 microM). Targets protein phosphatase 1alpha (PP1alpha) and reduces hydrogen peroxide. Corrects aberrant splicing of the muscle chloride channel Clcn1 in myotonic dystrophy type 1 (DM1). Shows potential anti-inflammatory activity.
- SMILESCCCC[C@@H](C)\C=C(/C)\C=C(/C)C(=O)NC1=C[C@@](O)(\C=C\C=C\C=C\C(=O)NC2=C(O)CCC2=O)[C@@H]2O[C@@H]2C1=O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200


![Manumycin A [52665-74-4]](https://www.targetmol.com/group3/M00/37/FC/CgoaEWayVNGEJykJAAAAAK5PwAs645.png)