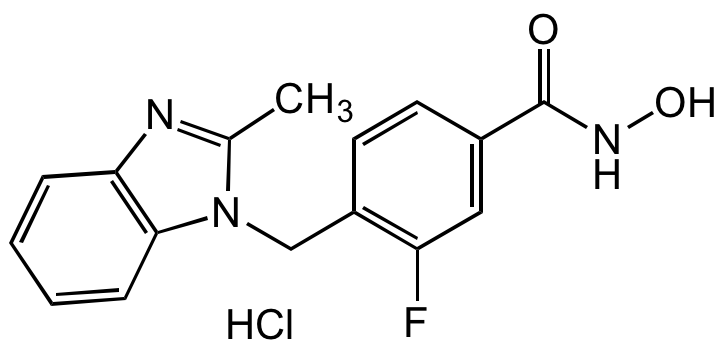
Chemical Structure
MBIMPH F-Analog 2
AG-CR1-3909
Estimated Purity>95%
Product group Chemicals
Molecular Weight299.3
Overview
- SupplierAdipoGen Life Sciences
- Product NameMBIMPH F-Analog 2
- Delivery Days Customer10
- CertificationResearch Use Only
- Estimated Purity>95%
- Molecular FormulaC16H14FN3O2
- Molecular Weight299.3
- Scientific DescriptionCell permeable, potent and selective class IIb HDAC6 inhibitor (IC50 =5nM). Displays high selectivity over all other HDACs (IC50=0.2-11microM). Induces cellular alpha-tubulin, but not histone H3 hyperacetylation in Neuro-2a cells. Promotes mitochondrial transport. Shows improved kinetics and biochemical potency against HDAC6 compared to tubastatin A (AG-CR1-3900). HDAC6 deacetylates tubulin, HSP90 and the core histones (H2A, H2B, H3, H4). Histone deacetylases act via the formation of large multiprotein complexes. HDAC6 plays an important role in microtubule-dependent cell motility, transcriptional regulation, degradation of misfolded proteins and cell cycle and is involved in autophagy, inflammation, cancer and neurodegeneration. - Chemical. Formula: C16H14FN3O2. MW: 299.3. Cell permeable, potent and selective class IIb HDAC6 inhibitor (IC50 =5nM). Displays high selectivity over all other HDACs (IC50=0.2-11microM). Induces cellular alpha-tubulin, but not histone H3 hyperacetylation in Neuro-2a cells. Promotes mitochondrial transport. Shows improved kinetics and biochemical potency against HDAC6 compared to tubastatin A (AG-CR1-3900). HDAC6 deacetylates tubulin, HSP90 and the core histones (H2A, H2B, H3, H4). Histone deacetylases act via the formation of large multiprotein complexes. HDAC6 plays an important role in microtubule-dependent cell motility, transcriptional regulation, degradation of misfolded proteins and cell cycle and is involved in autophagy, inflammation, cancer and neurodegeneration.
- SMILESCC1=NC2=C(C=C(F)C=C2)N1CC1=CC=C(C=C1)C(=O)NO
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200