Chemical Structure
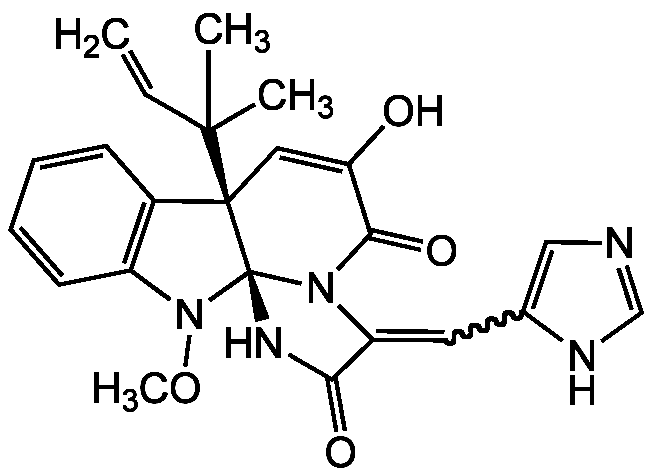
Meleagrin [71751-77-4] [71751-77-4]
AG-CN2-0451
CAS Number71751-77-4
Product group Chemicals
Estimated Purity>90%
Molecular Weight433.5
Overview
- SupplierAdipoGen Life Sciences
- Product NameMeleagrin [71751-77-4] [71751-77-4]
- Delivery Days Customer10
- CAS Number71751-77-4
- CertificationResearch Use Only
- Estimated Purity>90%
- Molecular FormulaC23H23N5O4
- Molecular Weight433.5
- Scientific DescriptionChemical. CAS: 71751-77-4. Formula: C23H23N5O4. MW: 433.5. Isolated from Penicillium sp. Mycotoxin. Alkaloid antibiotic. Bacterial enoyl-acyl carrier protein reductase (FabI) inhibitor. Antineoplastic activity. Moderate cytotoxicity against A-549 and HL-60 cell lines. Induces cell cycle arrest through G2/M phase, presumably by inhibiting tubulin polymerization. Antifouling agent against the barnacle Balanus amphitrite. - Mycotoxin. Alkaloid antibiotic. Bacterial enoyl-acyl carrier protein reductase (FabI) inhibitor. Antineoplastic activity. Moderate cytotoxicity against A-549 and HL-60 cell lines. Induces cell cycle arrest through G2/M phase, presumably by inhibiting tubulin polymerization. Antifouling agent against the barnacle Balanus amphitrite.
- SMILESCON1C2=C(C=CC=C2)[C@@]2(C=C(O)C(=O)N3C(=CC4=CN=CN4)C(=O)N[C@@]123)C(C)(C)C=C
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200

![Meleagrin [71751-77-4]](https://www.targetmol.com/group3/M00/35/8A/CgoaEWayI-OEAA0JAAAAANPeqU0752.png)