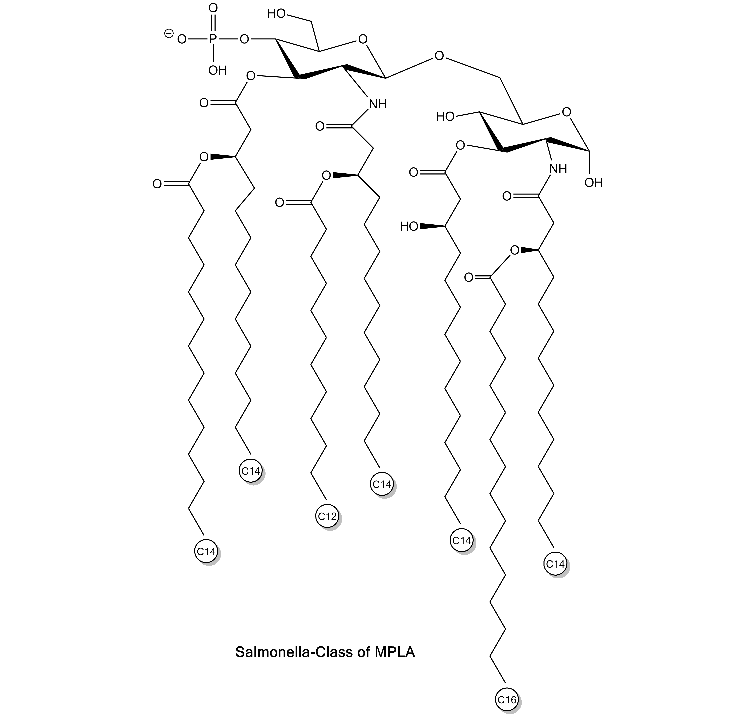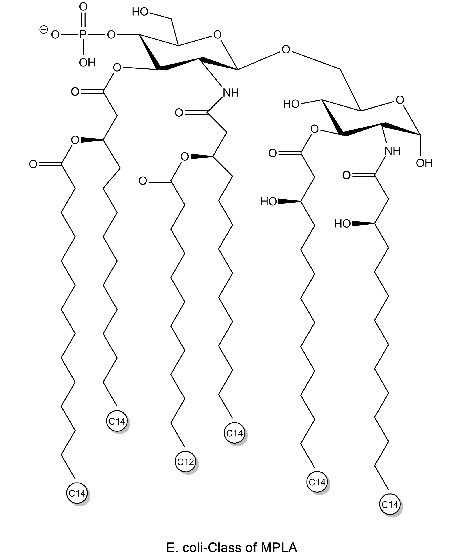
Chemical Structure
Monophosphoryl Lipid A [MPLA] from E. coli R515 (Re) TLRpure Sterile Solution
IAX-100-003
Estimated Purity>99.9%
Product group Chemicals
Overview
- SupplierInnaxon
- Product NameMonophosphoryl Lipid A [MPLA] from E. coli R515 (Re) TLRpure Sterile Solution
- Delivery Days Customer2
- CertificationResearch Use Only
- Concentration1 mg/ml
- Estimated Purity>99.9%
- Hazard InformationWarning
- Scientific DescriptionActivation of cells by LPS is mediated by the Toll-like receptor 4 (TLR4), a member of the highly conserved protein family of TLRs, which are specialised in the recognition of microbial components. In mice, defects in TLR4 result in LPS unresponsiveness. For optimal interaction with LPS, TLR4 requires association with myeloid differentiation protein 2 (MD-2). According to current consensus activation of TLR4 is preceded by the transfer of LPS to membrane-bound (m) or soluble (s) CD14 by LPS-binding protein (LBP). This mechanism is believed to be generally true for LPS signaling. Re-form LPS and lipid A, but not S-form LPS, are capable of inducing TNF-alpha responses also in the absence of CD14. LPS, synthesized by most wild-type (WT) Gram-negative bacteria (S-form LPS), consists of three regions, the O-polysaccharide chain, which is made up of repeating oligosaccharide units, the core oligosaccharide and the lipid A, which harbors the endotoxic activity of the entire molecule. R-form LPS synthesized by the so-called rough (R) mutants of Gram-negative bacteria lacks the O-specific chain. Furthermore, the core-oligosaccharide may be present in different degrees of completion, depending on the class (Ra to Re) to which the mutant belongs. Monophosphoryl Lipid A (MPLA) represents a detoxified derivative of Lipid A and constitutes an important adjuvant in prophylactic and therapeutic vaccines. - Chemical. Isolated and purified from E. coli strain R515. Activation of cells by LPS is mediated by the Toll-like receptor 4 (TLR4), a member of the highly conserved protein family of TLRs, which are specialised in the recognition of microbial components. In mice, defects in TLR4 result in LPS unresponsiveness. For optimal interaction with LPS, TLR4 requires association with myeloid differentiation protein 2 (MD-2). According to current consensus activation of TLR4 is preceded by the transfer of LPS to membrane-bound (m) or soluble (s) CD14 by LPS-binding protein (LBP). This mechanism is believed to be generally true for LPS signaling. Re-form LPS and lipid A, but not S-form LPS, are capable of inducing TNF-alpha responses also in the absence of CD14. LPS, synthesized by most wild-type (WT) Gram-negative bacteria (S-form LPS), consists of three regions, the O-polysaccharide chain, which is made up of repeating oligosaccharide units, the core oligosaccharide and the lipid A, which harbors the endotoxic activity of the entire molecule. R-form LPS synthesized by the so-called rough (R) mutants of Gram-negative bacteria lacks the O-specific chain. Furthermore, the core-oligosaccharide may be present in different degrees of completion, depending on the class (Ra to Re) to which the mutant belongs. Monophosphoryl Lipid A (MPLA) represents a detoxified derivative of Lipid A and constitutes an important adjuvant in prophylactic and therapeutic vaccines.
- Storage Instruction2°C to 8°C
- UNSPSC12352200