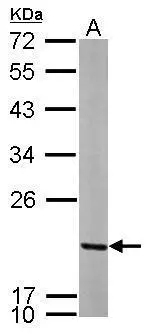
![Whole cell extract (30 μg) was separated by 12% SDS-PAGE, and the membrane was blotted with MRPL12 antibody [N1C3] (GTX114731) diluted at 1:1000. The HRP-conjugated anti-rabbit IgG antibody (GTX213110-01) was used to detect the primary antibody. Whole cell extract (30 μg) was separated by 12% SDS-PAGE, and the membrane was blotted with MRPL12 antibody [N1C3] (GTX114731) diluted at 1:1000. The HRP-conjugated anti-rabbit IgG antibody (GTX213110-01) was used to detect the primary antibody.](https://www.genetex.com/upload/website/prouct_img/normal/GTX114731/GTX114731_40233_20220708_WB_22071401_578.webp)
Whole cell extract (30 μg) was separated by 12% SDS-PAGE, and the membrane was blotted with MRPL12 antibody [N1C3] (GTX114731) diluted at 1:1000. The HRP-conjugated anti-rabbit IgG antibody (GTX213110-01) was used to detect the primary antibody.
MRPL12 antibody [N1C3]
GTX114731
ApplicationsImmunoFluorescence, Western Blot, ImmunoCytoChemistry, ImmunoHistoChemistry, ImmunoHistoChemistry Paraffin, Other Application
Product group Antibodies
ReactivityHuman, Mouse
TargetMRPL12
Overview
- SupplierGeneTex
- Product NameMRPL12 antibody [N1C3]
- Delivery Days Customer9
- Application Supplier NoteWB: 1:500-1:3000. ICC/IF: 1:100-1:1000. IHC-P: 1:100-1:1000. *Optimal dilutions/concentrations should be determined by the researcher.Not tested in other applications.
- ApplicationsImmunoFluorescence, Western Blot, ImmunoCytoChemistry, ImmunoHistoChemistry, ImmunoHistoChemistry Paraffin, Other Application
- CertificationResearch Use Only
- ClonalityPolyclonal
- Concentration0.56 mg/ml
- ConjugateUnconjugated
- Gene ID6182
- Target nameMRPL12
- Target descriptionmitochondrial ribosomal protein L12
- Target synonyms5c5-2, L12mt, MRP-L31/34, MRPL7, MRPL7/L12, RPML12, bL12m, large ribosomal subunit protein bL12m, 39S ribosomal protein L12, mitochondrial, MRP-L12, mitochondrial large ribosomal subunit protein bL12m
- HostRabbit
- IsotypeIgG
- Protein IDP52815
- Protein NameLarge ribosomal subunit protein bL12m
- Scientific DescriptionMammalian mitochondrial ribosomal proteins are encoded by nuclear genes and help in protein synthesis within the mitochondrion. Mitochondrial ribosomes (mitoribosomes) consist of a small 28S subunit and a large 39S subunit. They have an estimated 75% protein to rRNA composition compared to prokaryotic ribosomes, where this ratio is reversed. Another difference between mammalian mitoribosomes and prokaryotic ribosomes is that the latter contain a 5S rRNA. Among different species, the proteins comprising the mitoribosome differ greatly in sequence, and sometimes in biochemical properties, which prevents easy recognition by sequence homology. This gene encodes a 39S subunit protein which forms homodimers. In prokaryotic ribosomes, two L7/L12 dimers and one L10 protein form the L8 protein complex. [provided by RefSeq]
- ReactivityHuman, Mouse
- Storage Instruction-20°C or -80°C,2°C to 8°C
- UNSPSC41116161

![MRPL12 antibody [N1C3] detects MRPL12 protein at mitochondria by immunofluorescent analysis. Sample: HeLa cells were fixed in 2% paraformaldehyde/culture medium at 37oC for 30 min. Green: MRPL12 protein stained by MRPL12 antibody [N1C3] (GTX114731) diluted at 1:500. Blue: Hoechst 33342 staining. MRPL12 antibody [N1C3] detects MRPL12 protein at mitochondria by immunofluorescent analysis. Sample: HeLa cells were fixed in 2% paraformaldehyde/culture medium at 37oC for 30 min. Green: MRPL12 protein stained by MRPL12 antibody [N1C3] (GTX114731) diluted at 1:500. Blue: Hoechst 33342 staining.](https://www.genetex.com/upload/website/prouct_img/normal/GTX114731/GTX114731_40233_IFA_w_23060518_330.webp)