Chemical Structure
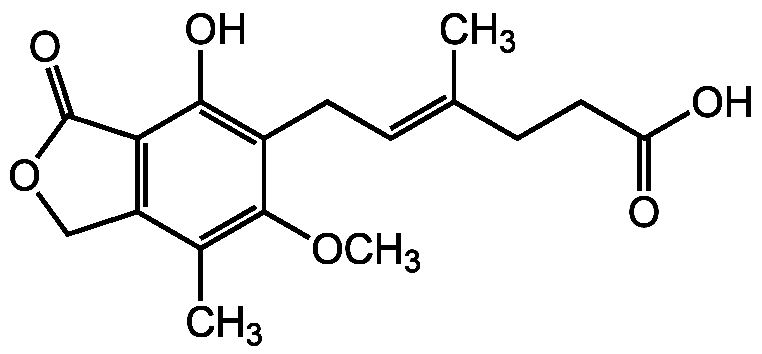
Mycophenolic acid [24280-93-1] [24280-93-1]
AG-CN2-0419
CAS Number24280-93-1
Product group Chemicals
Estimated Purity>98%
Molecular Weight320.3
Overview
- SupplierAdipoGen Life Sciences
- Product NameMycophenolic acid [24280-93-1] [24280-93-1]
- Delivery Days Customer10
- CAS Number24280-93-1
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationDanger,Excepted quantity
- Molecular FormulaC17H20O6
- Molecular Weight320.3
- Scientific DescriptionAntibiotic [1]. Shows antiviral, antifungal and antitumor properties [1, 2]. Immunosuppressive drug used to prevent rejection in organ transplantation, rheumatoid arthritis, and psoriasis [3-6, 13]. Potent reversible inhibitor of inosine-5-monophosphate dehydrogenase (IMPDH), leading to depletion of GMP and interruption of the de novo synthesis of purine nucleotides necessary for B and T lymphocyte proliferation [7, 8]. Inhibits the type II IMPDH isoform (IMPDH-2) 5-fold more potently compared to type I isoform [11]. Inhibits RNA and DNA synthesis [13]. Inducible nitric oxide synthase (iNOS/NOS II) inhibitor [8, 10]. Apoptosis and necrosis inducer [9, 12]. Novel type of inhibitor against RNA guanylyltransferases [14]. Inhibits TNF-alpha-stimulated MAPK/NF-kappaB and ROS generation [15]. Autophagy suppressor. Broad-spectrum antiviral compound with inhibitory activity against different coronavirus, including SARS-CoV, MERS-CoV and SARS-CoV-2, cause of COVID-19. It binds to the active site of SARS-CoV-2 papain-like protease (PLpro) possibly inhibiting viral replication. - Chemical. CAS: 24280-93-1. Formula: C17H20O6. MW: 320.3. Isolated from Penicillium brevi-compactum. Antibiotic. Shows antiviral, antifungal and antitumor properties. Immunosuppressive drug used to prevent rejection in organ transplantation, rheumatoid arthritis, and psoriasis. Potent reversible inhibitor of inosine-5-monophosphate dehydrogenase (IMPDH), leading to depletion of GMP and interruption of the de novo synthesis of purine nucleotides necessary for B and T lymphocyte proliferation. Inhibits the type II IMPDH isoform (IMPDH-2) 5-fold more potently compared to type I isoform. Inhibits RNA and DNA synthesis. Inducible nitric oxide synthase (iNOS/NOS II) inhibitor. Apoptosis and necrosis inducer. Novel type of inhibitor against RNA guanylyltransferases. Inhibits TNF-alpha-stimulated MAPK/NF-kappaB and ROS generation.
- SMILESCOC1=C(C)C2=C(C(=O)OC2)C(O)=C1C\C=C(/C)CCC(O)=O
- Storage Instruction2°C to 8°C,RT
- UN NumberUN 3077
- UNSPSC12352200


![Mycophenolic acid [24280-93-1] [24280-93-1]](https://www.targetmol.com/group3/M00/03/25/CgoaEWY7QvSEGW05AAAAAEXgGm0837.png)