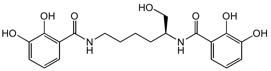
Chemical Structure
Myxochelin A [120243-02-9] [120243-02-9]
AG-CN2-0470
CAS Number120243-02-9
Product group Chemicals
Estimated Purity>99%
Molecular Weight404.4
Overview
- SupplierAdipoGen Life Sciences
- Product NameMyxochelin A [120243-02-9] [120243-02-9]
- Delivery Days Customer10
- CAS Number120243-02-9
- CertificationResearch Use Only
- Estimated Purity>99%
- Hazard InformationWarning
- Molecular FormulaC20H24N2O7
- Molecular Weight404.4
- Scientific DescriptionChemical. CAS: 120243-02-9. Formula: C20H24N2O7. MW: 404.4. Synthetic. Originally isolated from Pyxidicoccus fallax HKI 727. Potent inhibitor of human 5-lipoxygenase (5-LO). This enzyme catalyzes two initial steps in the conversion of arachidonic acid into leukotrienes, well known mediators of inflammatory and allergic reactions. Iron-chelating compound. Anticancer antibiotic. Shown to inhibit tumor cell invasion in vitro. Antibacterial compound. Antioxidant with free radical scavenging activities. - Potent inhibitor of human 5-lipoxygenase (5-LO). This enzyme catalyzes two initial steps in the conversion of arachidonic acid into leukotrienes, well known mediators of inflammatory and allergic reactions. Iron-chelating compound. Anticancer antibiotic. Shown to inhibit tumor cell invasion in vitro. Antibacterial compound. Antioxidant with free radical scavenging activities.
- SMILESOC[C@H](CCCCNC(=O)C1=C(O)C(O)=CC=C1)NC(=O)C1=CC=CC(O)=C1O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200

![Myxochelin A [120243-02-9] [120243-02-9]](https://www.targetmol.com/group3/M00/02/7F/CgoaEWY7L46ELZykAAAAAMOSpCw124.png)