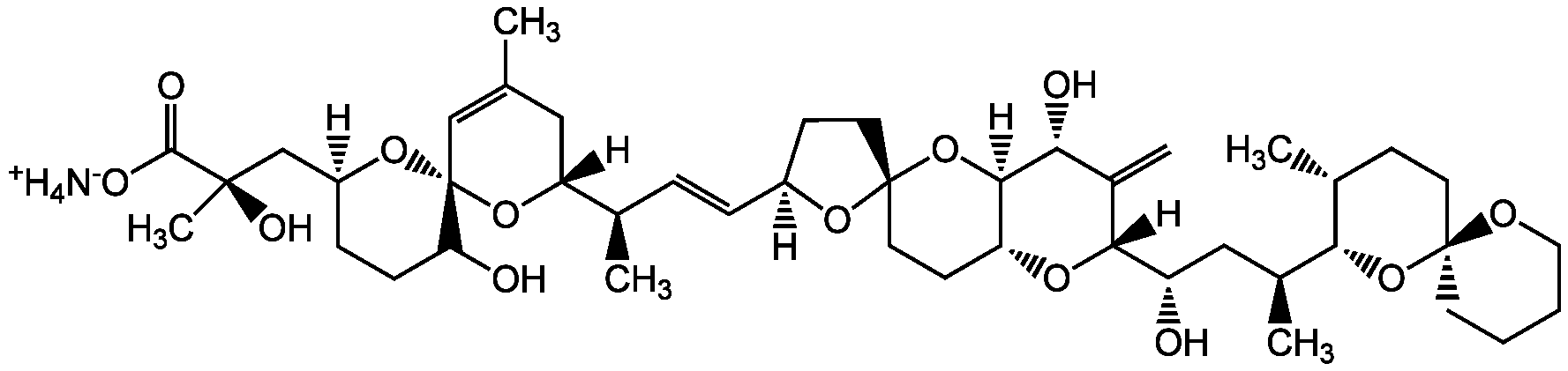
Chemical Structure
Okadaic acid . ammonium salt (high purity) [175522-42-6] [175522-42-6]
AG-CN2-0058
CAS Number175522-42-6
Product group Chemicals
Estimated Purity>98%
Molecular Weight804.0 . 18.0
Overview
- SupplierAdipoGen Life Sciences
- Product NameOkadaic acid . ammonium salt (high purity) [175522-42-6] [175522-42-6]
- Delivery Days Customer10
- CAS Number175522-42-6
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationDanger,Excepted quantity
- Molecular FormulaC44H67O13 . NH4
- Molecular Weight804.0 . 18.0
- Scientific DescriptionChemical. CAS: 175522-42-6. Formula: C44H67O13 . NH4. MW: 804.0 . 18.0. Isolated from Prorocentrum concavum. Salt form generated in aqueous ammonium hydroxide-methanol solution. Non-phorbol type tumor promoter. Reversible, potent and selective serine threonine protein phosphatase inhibitor. PP2A (IC50=0.2-1nM), PP1 (IC50=3-15nM), PP2B (IC50= >1microM). Does not inhibit PP2C. Stimulates intracellular protein phosphorylation. Useful tool for studying cellular processes that are regulated by phosphorylation. Does not affect activity of acid phosphatase, alkaline phosphatase and tyrosine phosphatase. Mimics the effects of insulin. Activates atypical protein kinase C (zeta/lambda) in 3T3/L1 adipocytes. Enhances transmitter release at neuromuscular junctions. Apoptosis inhibitor. Induces apoptosis in human breast carcinoma cells (MB-231 and MCF-7) and in myeloid cells. Neurotoxic. Used to study various cellular processes including cell cycle, apoptosis, nitric oxide metabolism and calcium signaling. Stimulates cell motility, loss of stabilization of focal adhesions and a consequent loss of cytoskeletal organization. - Non-phorbol type tumor promoter [1]. Reversible, potent and selective serine threonine protein phosphatase inhibitor. PP2A (IC50=0.2-1nM), PP1 (IC50=3-15nM), PP2B (IC50= >1microM). Does not inhibit PP2C [2, 6, 7, 18]. Stimulates intracellular protein phosphorylation [3]. Useful tool for studying cellular processes that are regulated by phosphorylation. Does not affect activity of acid phosphatase, alkaline phosphatase and tyrosine phosphatase [4, 5]. Mimics the effects of insulin [7]. Activates atypical protein kinase C (zeta/lambda) in 3T3/L1 adipocytes [14, 19]. Enhances transmitter release at neuromuscular junctions [8]. Apoptosis inhibitor [9, 11, 12]. Induces apoptosis in human breast carcinoma cells (MB-231 and MCF-7) and in myeloid cells [10]. Neurotoxic [12, 16]. Used to study various cellular processes including cell cycle, apoptosis, nitric oxide metabolism and calcium signaling [13, 15, 17, 18]. Stimulates cell motility, loss of stabilization of focal adhesions and a consequent loss of cytoskeletal organization [20].
- SMILES[NH4+].[H][C@@]1(CC[C@@]2(CC[C@H]3O[C@@]([H])([C@@H](O)C[C@H](C)[C@H]4O[C@@]5(CCCCO5)CC[C@H]4C)C(=C)[C@@H](O)[C@]3([H])O2)O1)\C=C\[C@@H](C)[C@@]1([H])CC(C)=C[C@@]2(O[C@]([H])(C[C@@](C)(O)C([O-])=O)CCC2O)O1
- Storage Instruction-20°C,2°C to 8°C
- UN NumberUN 3462
- UNSPSC12352200

![Okadaic acid ammonium salt [175522-42-6] [175522-42-6]](https://www.targetmol.com/group3/M00/02/F1/CgoaEWY7PS2EbUCJAAAAAAj6LnQ172.png)