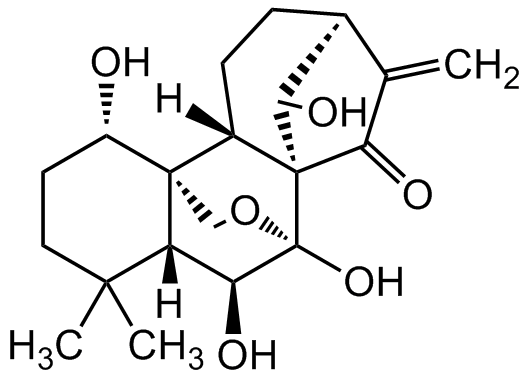
Chemical Structure
Oridonin [28957-04-2]
CDX-O0131
CAS Number28957-04-2
Product group Chemicals
Estimated Purity>98%
Molecular Weight364.43
Overview
- SupplierChemodex
- Product NameOridonin [28957-04-2]
- Delivery Days Customer2
- CAS Number28957-04-2
- CertificationResearch Use Only
- Estimated Purity>98%
- Molecular FormulaC20H28O6
- Molecular Weight364.43
- Scientific DescriptionChemical. CAS: 28957-04-2. Formula: C20H28O6. MW: 364.43. Oridonin is a diterpenoid that has been found in R. rubescens and has anti-inflammatory and anticancer, antimicrobial and neuroprotective properties. The regulatory anticancer mechanisms include induction of apoptosis and autophagy, inhibition of proliferation, inhibition of angiogenesis, cell cycle arrest. It is an inhibitor of the TLR4/p38-MAPK and TLR4/NF-kappa signaling pathways and PPARgamma. Oridonin is a high affinity inhibitor of NLRP3 inflammasome assembly and activation (Kd = 52.5 nM). It inhibits inflammation in wild-type, but not Nlrp3-/-, mice in a model of high-fat diet-induced type 2 diabetes. Also inhibits glial activation, decreases inflammatory cytokine release, attenuates synaptic loss and improves behavioural deficits in Abeta1-42 treated mice. - Oridonin is a diterpenoid that has been found in R. rubescens and has anti-inflammatory and anticancer, antimicrobial and neuroprotective properties. The regulatory anticancer mechanisms include induction of apoptosis and autophagy, inhibition of proliferation, inhibition of angiogenesis, cell cycle arrest. It is an inhibitor of the TLR4/p38-MAPK and TLR4/NF-kappa signaling pathways and PPARgamma. Oridonin is a high affinity inhibitor of NLRP3 inflammasome assembly and activation (Kd = 52.5 nM). It inhibits inflammation in wild-type, but not Nlrp3-/-, mice in a model of high-fat diet-induced type 2 diabetes. Also inhibits glial activation, decreases inflammatory cytokine release, attenuates synaptic loss and improves behavioural deficits in Abeta1-42 treated mice. CRL/SCF RING E3 inhibitor. Inhibits Fbw7 an E3 ubiquitin ligase (CRL/SCF RING) of c-Myc and promotes proteasomal degradation.
- SMILESO[C@@H]1[C@]2(CO3)[C@@]([C@H](O)[C@]3(O)[C@@](C4=O)([C@@H]5O)[C@@]2([H])CC[C@H]5C4=C)([H])C(C)(C)CC1
- Storage Instruction2°C to 8°C,RT
- UNSPSC12352200


![Oridonin [28957-04-2]](https://www.targetmol.com/group3/M00/37/AB/CgoaEWayUDWEIW-wAAAAANO_Pnk366.png)