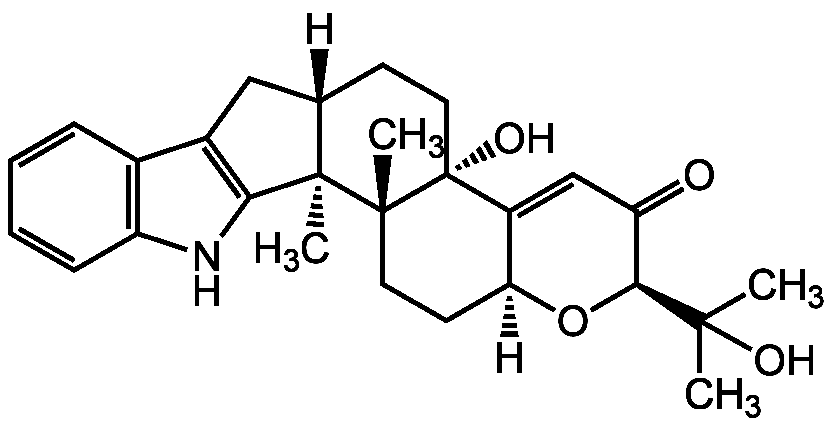
Chemical Structure
Paxilline [57186-25-1] [57186-25-1]
AG-CN2-0167
CAS Number57186-25-1
Product group Chemicals
Estimated Purity>95%
Molecular Weight435.6
Overview
- SupplierAdipoGen Life Sciences
- Product NamePaxilline [57186-25-1] [57186-25-1]
- Delivery Days Customer10
- CAS Number57186-25-1
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationDanger,Excepted quantity
- Molecular FormulaC27H33NO4
- Molecular Weight435.6
- Scientific DescriptionChemical. CAS: 57186-25-1. Formula: C27H33NO4. MW: 435.6. Isolated from Penicillium sp. Tremorgenic. Potent, selective and reversible inhibitor of high-conductance calcium-activated potassium (BKCa) channels. Reversible inhibitor of inositol 1,4,5-trisphosphate receptor (InsP(3)). Sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) inhibitor. Liver X receptor (LXRalpha and LXRbeta; NR1H3 and NR1H2) agonist in biochemical and in vitro cell-based assays. Anticonvulsant. Apoptosis enhancer. Can sensitize various glioma cells to TRAIL-mediated apoptosis. Shown to protect neuronal cells against glutamate-induced cell death. - Tremorgenic [1]. Potent, selective and reversible inhibitor of high-conductance calcium-activated potassium (BKCa) channels [2]. Reversible inhibitor of inositol 1,4,5-trisphosphate receptor (InsP(3)) [3]. Sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) inhibitor [4]. Liver X receptor (LXRalpha and LXRbeta; NR1H3 and NR1H2) agonist in biochemical and in vitro cell-based assays [5]. Anticonvulsant [6]. Apoptosis enhancer. Can sensitize various glioma cells to TRAIL-mediated apoptosis [7]. Shown to protect neuronal cells against glutamate-induced cell death [8].
- SMILES[H][C@]12CC3=C(NC4=C3C=CC=C4)[C@]1(C)[C@@]1(C)CC[C@]3([H])O[C@@H](C(=O)C=C3[C@]1(O)CC2)C(C)(C)O
- Storage Instruction-20°C,2°C to 8°C
- UN NumberUN 2811
- UNSPSC12352200


![Paxilline [57186-25-1]](https://www.targetmol.com/group3/M00/36/E0/CgoaEGayRNiEb7-nAAAAAHHSybc253.png)