Chemical Structure
Phomopsin A [64925-80-0] [64925-80-0]
AG-CN2-0515
CAS Number64925-80-0
Product group Chemicals
Estimated Purity>98%
Molecular Weight789.2
Overview
- SupplierAdipoGen Life Sciences
- Product NamePhomopsin A [64925-80-0] [64925-80-0]
- Delivery Days Customer10
- CAS Number64925-80-0
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationWarning
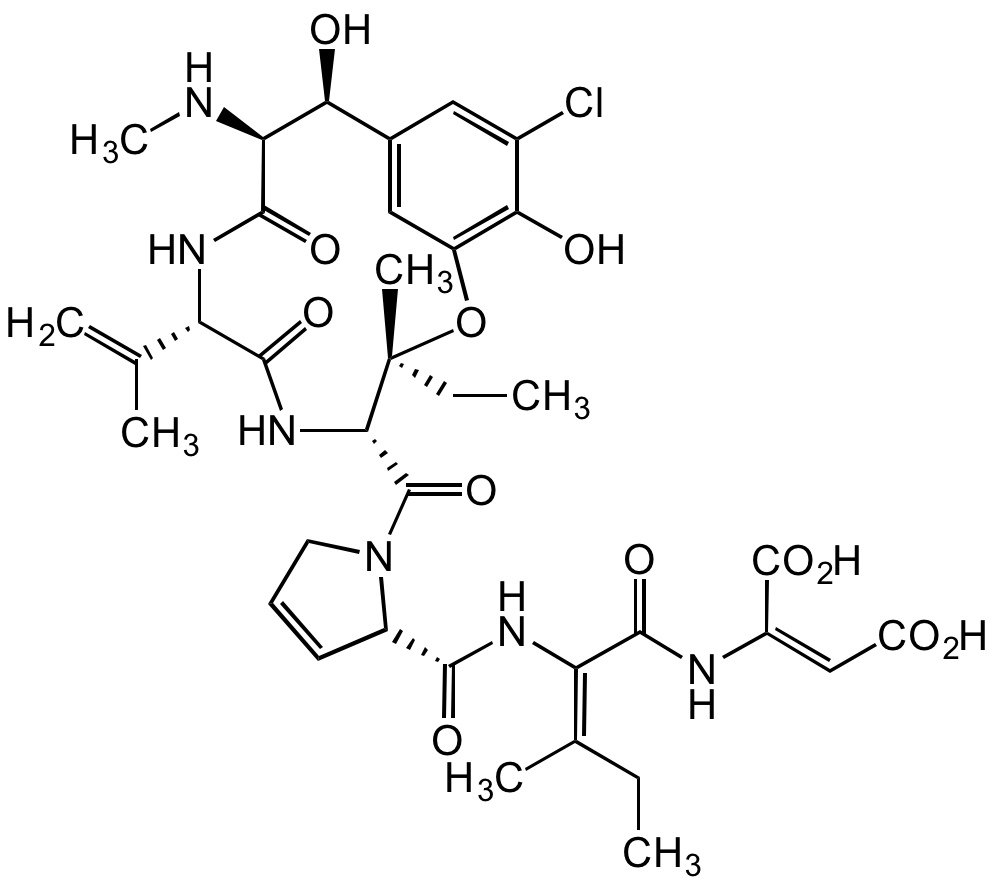
- Molecular FormulaC36H45ClN6O12
- Molecular Weight789.2
- Scientific DescriptionChemical. CAS: 64925-80-0. Formula: C36H45ClN6O12. MW: 789.2. Isolated from Phomopsis leptostromiformis. Macrocyclic heptapeptide mycotoxin. Potent anti-mitotic compound that can cause cell death. Microtubule assembly inhibitor. Binds selectively to dimeric tubulin, inhibiting the formation of the microtubule spindle to block cell division. Binds at a site different from the colchicine binding site and overlapping the vinblastine binding site. Inhibits tubulin-dependent GTP hydrolysis. Binds beta-tubulin from higher organisms but not alpha-tubulin or fungal mycelial tubulin. Causes lupinosis (a degenerative disorder) in livestock fed infected lupins. - Macrocyclic heptapeptide mycotoxin. Potent anti-mitotic compound that can cause cell death. Microtubule assembly inhibitor. Binds selectively to dimeric tubulin, inhibiting the formation of the microtubule spindle to block cell division. Binds at a site different from the colchicine binding site and overlapping the vinblastine binding site. Inhibits tubulin-dependent GTP hydrolysis. Binds beta-tubulin from higher organisms but not alpha-tubulin or fungal mycelial tubulin. Causes lupinosis (a degenerative disorder) in livestock fed infected lupins.
- SMILESClC1=CC([C@H](O)[C@@H]2NC)=CC(O[C@@](C)(CC)[C@H](C(N3CC=C[C@H]3C(N/C(C(N/C(C(O)=O)=C/C(O)=O)=O)=C(C)/CC)=O)=O)NC([C@H](C(C)=C)NC2=O)=O)=C1O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200

![Phomopsin A [64925-80-0] [64925-80-0]](https://www.targetmol.com/group3/M00/36/E0/CgoaEGayRNqEW9kvAAAAAH63IQU836.png)