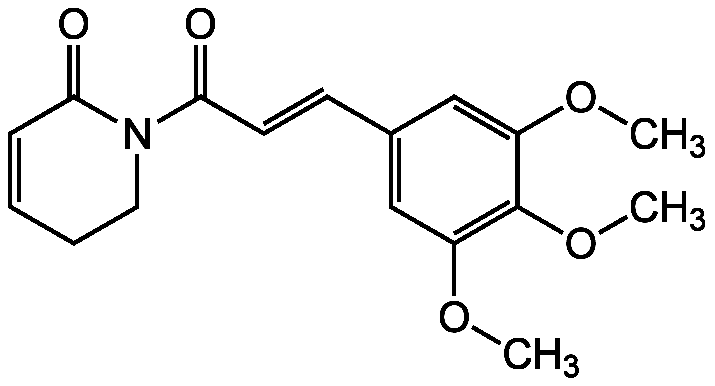
Chemical Structure
Piperlongumine
AG-CN2-0024
CAS Number20069-09-4
Product group Chemicals
Estimated Purity>97%
Molecular Weight317.3
Overview
- SupplierAdipoGen Life Sciences
- Product NamePiperlongumine
- Delivery Days Customer10
- CAS Number20069-09-4
- CertificationResearch Use Only
- Estimated Purity>97%
- Molecular FormulaC17H19NO5
- Molecular Weight317.3
- Scientific DescriptionChemical. CAS: 20069-09-4. Formula: C17H19NO5. MW: 317.3. Isolated from Piper longum roots. Cytotoxic against tumor cell lines. Induces necrosis and apoptosis in cancer cells. Shows anti-platelet aggregation activity possibly by inhibition of cyclooxgenase activity and a decrease in thromboxane A2 formation. Shows significant anxiolytic and antidepressant activities. Promotes adipogenesis of 3T3-L1 cells. Induces in vivo and in vitro mutagenicity in eukaryotic models. Selectively kills cancer cells by targeting the stress response to ROS. Shows in vitro schistosomicidal activity. - Cytotoxic against tumor cell lines [3, 4, 5]. Induces necrosis and apoptosis in cancer cells [5, 9, 12]. Shows anti-platelet aggregation activity possibly by inhibition of cyclooxgenase activity and a decrease in thromboxane A2 formation [4, 6, 10]. Shows significant anxiolytic and antidepressant activities [7]. Promotes adipogenesis of 3T3-L1 cells [8]. Induces in vivo and in vitro mutagenicity in eukaryotic models [11]. Selectively kills cancer cells by targeting the stress response to ROS [12]. Shows in vitro schistosomicidal activity [13]. Selective inhibitor of human immunoproteasome. Targets the beta5i subunit (LMP7) (IC50=15microM) with minimal inhibition of human constitutive proteasome [14].
- SMILESCOC1=CC(\C=C\C(=O)N2CCC=CC2=O)=CC(OC)=C1OC
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200



![Piperlongumine [20069-09-4]](https://www.targetmol.com/group3/M00/35/78/CgoaEGayICOEBOaVAAAAAJQFGuc308.png)