PSGL-1 (human):Fc (human) (rec.)
AG-40B-0190
Protein IDQ14242
Product group Proteins / Signaling Molecules
Overview
- SupplierAdipoGen Life Sciences
- Product NamePSGL-1 (human):Fc (human) (rec.)
- Delivery Days Customer10
- CertificationResearch Use Only
- Concentration1 mg/ml
- Estimated Purity>95%
- Gene ID6404
- Target nameSELPLG
- Target descriptionselectin P ligand
- Target synonymsCD162, CLA, PSGL-1, PSGL1, P-selectin glycoprotein ligand 1, cutaneous lymphocyte-associated associated antigen
- Protein IDQ14242
- Protein NameP-selectin glycoprotein ligand 1
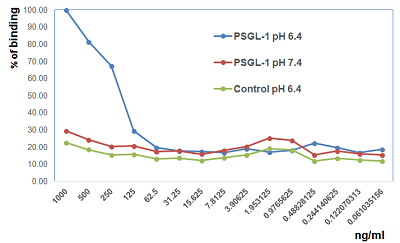
- Scientific DescriptionP-selectin glycoprotein ligand-1 (PSGL-1) is a 120kda transmembrane homodimer protein that is primarily expressed on lymphoid and myeloid cells, including platelets. PSGL-1 was first identified to regulate the rolling/tethering of neutrophils on activated endothelium through P-selectin binding. PSGL-1 binds P-, E- and L- selectin through the N-terminus of the extracellular domain upon special glycosylation (sialyl Lewis x). PSGL-1 functions in regulating the migration of macrophages/monocytes, plasma B cells, dendritic cells and T cells by selectin engagement. PSGL-1 also regulates localization of macrophages, dendritic cells and B cells in the lamina propria at steady state. Although PSGL-1 is expressed on resting T cells, selectin binding capacity is only acquired during the proliferation/differentiation of effector T cells. In addition to its migratory properties, the adhesion protein PSGL-1 also functions in a selectin-independent manner as a negative regulator of T cell responses in contexts of chronic viral infection, cancer and some autoimmune diseases. For this immune checkpoint function, it has recently been reported that PSGL-1 binds the negative immune checkpoint regulator VISTA (V-domain immunoglobulin suppressor of T cell activation). PSGL-1 expressed on leukocytes binds to multimeric VISTA at acidic pH, but not at the physiological pH 7. This acidic influence of the binding is due to multiple histidine residues protonation located in the VISTA extracellular domain. VISTA - PSGL-1 interaction shows that immune response can be regulated by acidic environments found in tumors. Identification of PSGL-1 - VISTA signaling pathways in T cells could lead to new therapeutic targets that increase immune function, such as in chronic viral infections or in cancer, or attenuate immune function such as in autoimmunity. - Protein. The extracellular domain of human PSGL-1 (aa 42-305) is fused at the C-terminus to the Fc portion of human IgG1. Source: HEK 293 cells. Endotoxin content: 95% (SDS-PAGE). P-selectin glycoprotein ligand-1 (PSGL-1) is a 120kda transmembrane homodimer protein that is primarily expressed on lymphoid and myeloid cells, including platelets. PSGL-1 was first identified to regulate the rolling/tethering of neutrophils on activated endothelium through P-selectin binding. PSGL-1 binds P-, E- and L- selectin through the N-terminus of the extracellular domain upon special glycosylation (sialyl Lewis x). PSGL-1 functions in regulating the migration of macrophages/monocytes, plasma B cells, dendritic cells and T cells by selectin engagement. PSGL-1 also regulates localization of macrophages, dendritic cells and B cells in the lamina propria at steady state. Although PSGL-1 is expressed on resting T cells, selectin binding capacity is only acquired during the proliferation/differentiation of effector T cells. In addition to its migratory properties, the adhesion protein PSGL-1 also functions in a selectin-independent manner as a negative regulator of T cell responses in contexts of chronic viral infection, cancer and some autoimmune diseases. For this immune checkpoint function, it has recently been reported that PSGL-1 binds the negative immune checkpoint regulator VISTA (V-domain immunoglobulin suppressor of T cell activation). PSGL-1 expressed on leukocytes binds to multimeric VISTA at acidic pH, but not at the physiological pH 7. This acidic influence of the binding is due to multiple histidine residues protonation located in the VISTA extracellular domain. VISTA - PSGL-1 interaction shows that immune response can be regulated by acidic environments found in tumors. Identification of PSGL-1 - VISTA signaling pathways in T cells could lead to new therapeutic targets that increase immune function, such as in chronic viral infections or in cancer, or attenuate immune function such as in autoimmunity.
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC41116100
- SpeciesHuman


