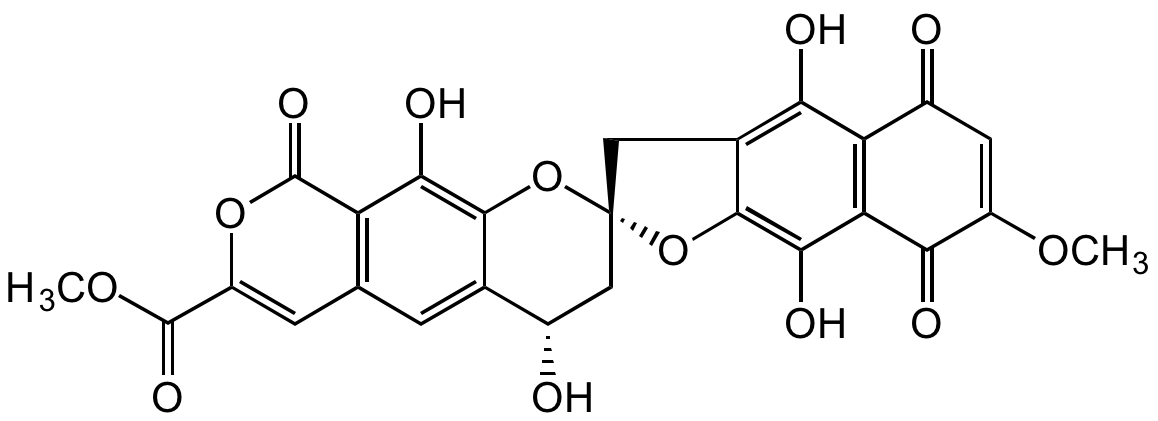
Chemical Structure
Purpuromycin [53969-01-0]
AG-CN2-0317
CAS Number53969-01-0
Product group Chemicals
Estimated Purity>95%
Molecular Weight538.4
Overview
- SupplierAdipoGen Life Sciences
- Product NamePurpuromycin [53969-01-0]
- Delivery Days Customer10
- CAS Number53969-01-0
- CertificationResearch Use Only
- Estimated Purity>95%
- Molecular FormulaC26H18O13
- Molecular Weight538.4
- Scientific DescriptionChemical. CAS: 53969-01-0. Formula: C26H18O13. MW: 538.4. Isolated from Actinoplanes sp. Rubromycin antibiotic. Antibacterial, antifungal and anti-protozoal agent. Active against Gram-positive bacteria, Gram-negative bacteria and fungi. Telomerase inhibitor (IC50=3microM). Protein synthesis inhibitor. Binds tRNAs with high affinity and specifically inhibits aminoacyl-tRNA formation consequent inhibition of protein synthesis in these microorganisms. Inhibits of all tRNA synthetases. Effectively inhibits bacterial translation and translation in eukaryotic cells. Modest inhibitor of retroviral reverse transcriptase. - Rubromycin antibiotic. Antibacterial, antifungal and anti-protozoal agent. Active against Gram-positive bacteria, Gram-negative bacteria and fungi. Telomerase inhibitor (IC50=3microM). Protein synthesis inhibitor. Binds tRNAs with high affinity and specifically inhibits aminoacyl-tRNA formation consequent inhibition of protein synthesis in these microorganisms. Inhibits of all tRNA synthetases. Effectively inhibits bacterial translation and translation in eukaryotic cells. Modest inhibitor of retroviral reverse transcriptase.
- SMILESO[C@H]1C[C@@]2(OC3=C(O)C(C(OC(C(OC)=O)=C4)=O)=C4C=C13)CC5=C(O)C(C(C=C(OC)C6=O)=O)=C6C(O)=C5O2
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
