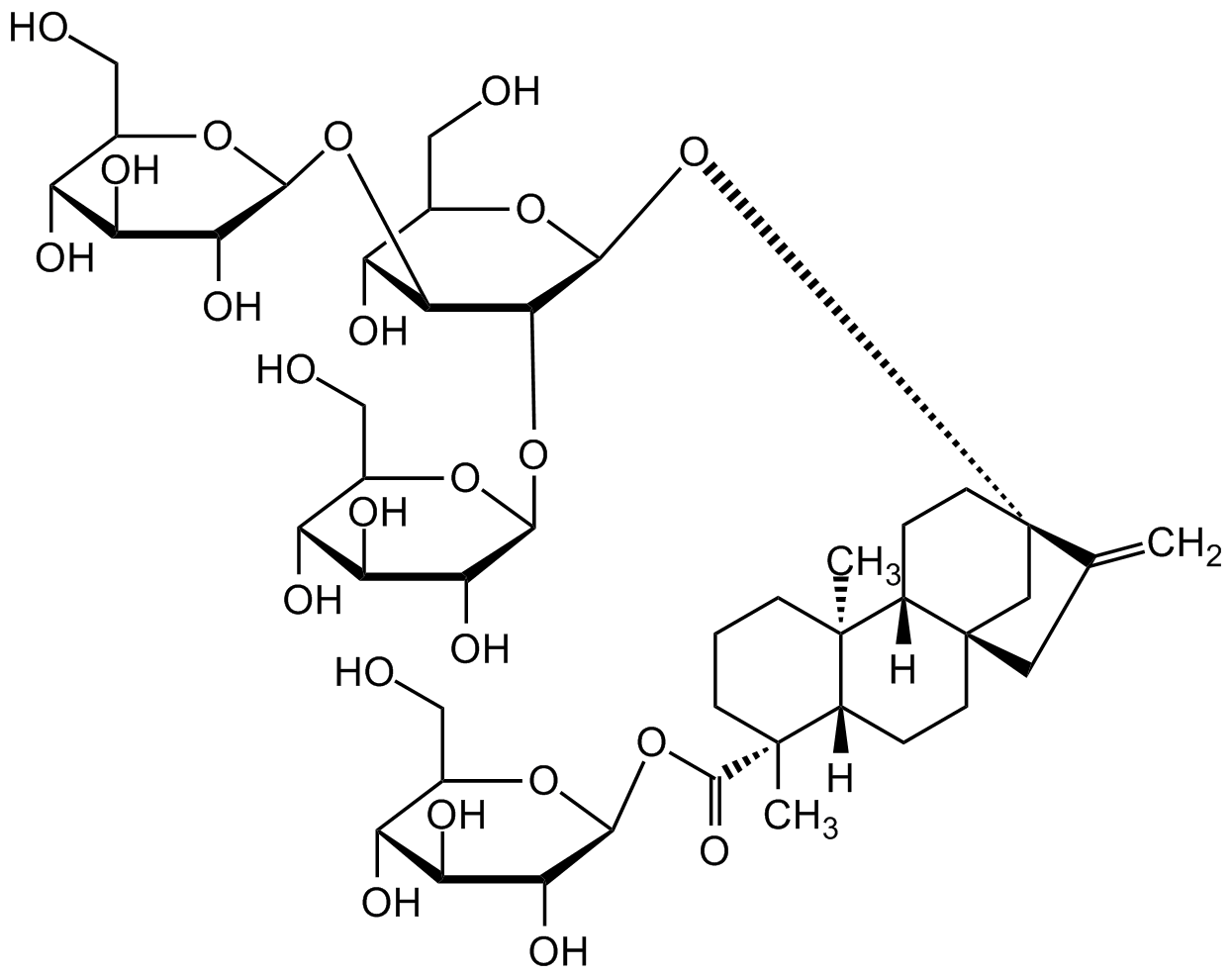
Chemical Structure
Rebaudioside A [58543-16-1] [58543-16-1]
CDX-R0090
CAS Number58543-16-1
Product group Chemicals
Estimated Purity>98%
Molecular Weight967.01
Overview
- SupplierChemodex
- Product NameRebaudioside A [58543-16-1] [58543-16-1]
- Delivery Days Customer2
- CAS Number58543-16-1
- CertificationResearch Use Only
- Estimated Purity>98%
- Molecular FormulaC44H70O23
- Molecular Weight967.01
- Scientific DescriptionChemical. CAS: 58543-16-1. Formula: C44H70O23. MW: 967.01. Rebaudioside A is a glucosylated steviol glycoside studied and used as a non-glycemic sweetener. It is one of the predominant steviol glycosides isolated from S. rebaudiana leaves. Rebaudioside A is a alpha-glucosidase inhibitor with (IC50=35microg/ml) and can inhibit ATP-sensitive K+-channels. In vitro rebaudioside A stimulated the insulin secretion from MIN6 cells in a dose- and glucose-dependent manner. It increases glucagon-like peptide 1 (GLP-1) secretion in a 2-dimensional mouse intestine model. Rebaudioside A is metabolized by gut microbiota to steviol. Rebaudioside A shows antioxidant activity on reducing cellular reactive oxygen species. It is an activator of Nrf2 and is a potential candidate hepatoprotective agent. - Rebaudioside A is a glucosylated steviol glycoside studied and used as a non-glycemic sweetener. It is one of the predominant steviol glycosides isolated from S. rebaudiana leaves. Rebaudioside A is a alpha-glucosidase inhibitor with (IC50=35microg/ml) and can inhibit ATP-sensitive K+-channels. In vitro rebaudioside A stimulated the insulin secretion from MIN6 cells in a dose- and glucose-dependent manner. It increases glucagon-like peptide 1 (GLP-1) secretion in a 2-dimensional mouse intestine model. Rebaudioside A is metabolized by gut microbiota to steviol. Rebaudioside A shows antioxidant activity on reducing cellular reactive oxygen species. It is an activator of Nrf2 and is a potential candidate hepatoprotective agent.
- SMILESC=C1[C@@]2(O[C@H]3[C@H](O[C@H]4[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O4)[C@@H](O[C@H]5[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O5)[C@H](O)[C@@H](CO)O3)C[C@]6(C1)CC[C@]7([H])[C@](C)(C(O[C@H]8[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O8)=O)CCC[C@@]7(C)[C@]6([H])CC2
- Storage InstructionRT
- UNSPSC12352200


![Rebaudioside A [58543-16-1]](https://www.targetmol.com/group3/M00/03/13/CgoaEGY7Rj-EJc6YAAAAAFIom6M998.png)