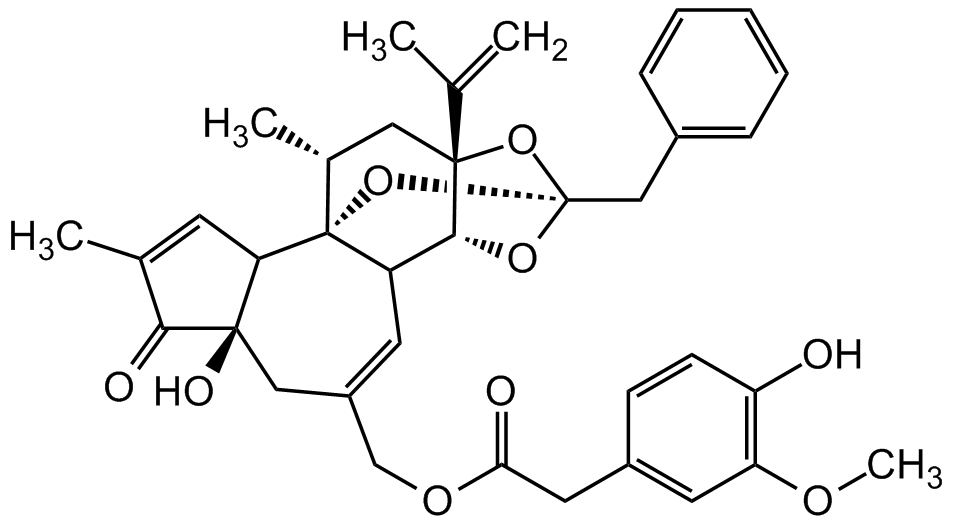
Chemical Structure
Resiniferatoxin (high purity) [57444-62-9] [57444-62-9]
AG-CN2-0534
CAS Number57444-62-9
Product group Chemicals
Estimated Purity>98%
Molecular Weight628.72
Overview
- SupplierAdipoGen Life Sciences
- Product NameResiniferatoxin (high purity) [57444-62-9] [57444-62-9]
- Delivery Days Customer10
- CAS Number57444-62-9
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationDanger,Excepted quantity
- Molecular FormulaC37H40O9
- Molecular Weight628.72
- Scientific DescriptionChemical. CAS: 57444-62-9. Formula: C37H40O9. MW: 628.72. Isolated from Euphorbia resinifera. Highly potent TRPV1 agonist. Resiniferatoxin (RTX) is a phorbol-related diterpene ester analog of capsaicin. RTX is a highly potent transient receptor potential vanilloid 1 (TRPV1) agonist (Ki=43pM) and acts as a selective modulator of primary afferent neurons. TRPV1 or vanilloid receptor 1 is a non-selective cation channel that is involved in the detection and transduction of nociceptive stimuli. TRPV1 is expressed in the plasma membrane of sensory neurons and stimulation by RTX causes this ion channel to become permeable to cations, especially calcium. The influx of cations causes the neuron to depolarize, transmitting signals similar to those that would be transmitted if the innervated tissue were being burned or damaged. This stimulation is followed by desensitization and analgesia, in part because the nerve endings die from calcium overload. Inflammation and nerve damage result in the up-regulation of TRPV1 transcription and therefore, modulators of TRPV1 channels are potentially useful in the treatment of inflammatory and neuropathic pain. The primary action of RTX is to activate sensory neurons responsible for the perception of pain. RTX has been developed as an analgesic (painkiller) as a mean to provide pain relief for forms of advanced cancer. RTX has been investigated for treatment of urinary bladder hyper-reflexia, interstitial cystitis, rhinitis, lifelong premature ejaculation and chronic pain conditions. RTX has also been shown anti-inflammatory activity in T. spiralis infection, by inhibiting the Th1 immune response through the inhibition of Th1 cytokines in the intestinal phase. - Highly potent TRPV1 agonist. Resiniferatoxin (RTX) is a phorbol-related diterpene ester analog of capsaicin. RTX is a highly potent transient receptor potential vanilloid 1 (TRPV1) agonist (Ki=43pM) and acts as a selective modulator of primary afferent neurons. TRPV1 or vanilloid receptor 1 is a non-selective cation channel that is involved in the detection and transduction of nociceptive stimuli. TRPV1 is expressed in the plasma membrane of sensory neurons and stimulation by RTX causes this ion channel to become permeable to cations, especially calcium. The influx of cations causes the neuron to depolarize, transmitting signals similar to those that would be transmitted if the innervated tissue were being burned or damaged. This stimulation is followed by desensitization and analgesia, in part because the nerve endings die from calcium overload. Inflammation and nerve damage result in the up-regulation of TRPV1 transcription and therefore, modulators of TRPV1 channels are potentially useful in the treatment of inflammatory and neuropathic pain. The primary action of RTX is to activate sensory neurons responsible for the perception of pain. RTX has been developed as an analgesic (painkiller) as a mean to provide pain relief for forms of advanced cancer. RTX has been investigated for treatment of urinary bladder hyper-reflexia, interstitial cystitis, rhinitis, lifelong premature ejaculation and chronic pain conditions. RTX has also been shown anti-inflammatory activity in T. spiralis infection, by inhibiting the Th1 immune response through the inhibition of Th1 cytokines in the intestinal phase.
- SMILESCC(C1=O)=CC2[C@]1(O)CC(COC(CC3=CC(OC)=C(O)C=C3)=O)=CC4[C@]25[C@H](C)C [C@@]6(C(C)=C)[C@@H]4O[C@@](O6)(CC7=CC=CC=C7)O5
- Storage Instruction-20°C,2°C to 8°C
- UN Number2811
- UNSPSC12352200

![Resiniferatoxin [57444-62-9]](https://www.targetmol.com/group3/M00/02/E8/CgoaEWY7PBOEW40vAAAAADlc4yY653.png)