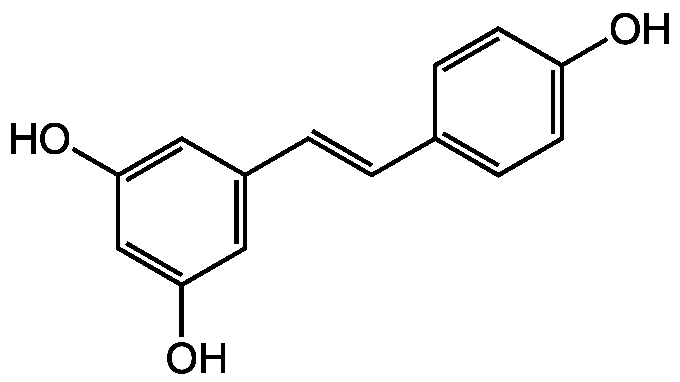
Chemical Structure
Resveratrol [501-36-0] [501-36-0]
AG-CN2-0033
CAS Number501-36-0
Product group Chemicals
Estimated Purity>98%
Molecular Weight228.2
Overview
- SupplierAdipoGen Life Sciences
- Product NameResveratrol [501-36-0] [501-36-0]
- Delivery Days Customer10
- CAS Number501-36-0
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationWarning
- Molecular FormulaC14H12O3
- Molecular Weight228.2
- Scientific DescriptionChemical. CAS: 501-36-0. Formula: C14H12O3. MW: 228.2. Isolated from Polygonum cuspidatum. Potent phenolic antioxidant found in grapes and red wine. Eicosanoid synthesis and platelet aggregation inhibitor. Estrogen receptor agonist. Chemopreventive. Specific inhibitor of cyclooxygenase-1 (COX-1). Anti-inflammatory. Ribonucleotide reductase and DNA synthesis. Arrests cell cycle at S/G2 phase. Anticancer and antiproliferative compound. Apoptosis inducer. Protein kinase D inhibitor. Does not inhibit PKC. Autophagy inducer. Potent SIRT1 (sirtuin 1) activator. Neuroprotective. Adipogenesis inhibitor. PGC-1alpha activator. Sonic hedgehog (Shh) signaling pathway modulator. Gli1 mRNA expression inhibitor. Downregulates Gli transcriptional activity. Cardioprotective. Anti-diabetic. Senescence modulator. Extends lifespan. Inhibitor of NLRP3 inflammasome activation. - Potent phenolic antioxidant found in grapes and red wine [1, 19]. Eicosanoid synthesis and platelet aggregation inhibitor [2]. Estrogen receptor agonist [3]. Chemopreventive [4, 15]. Specific inhibitor of cyclooxygenase-1 (COX-1) [4]. Anti-inflammatory [4, 21]. Ribonucleotide reductase and DNA synthesis [5]. Arrests cell cycle at S/G2 phase [6, 23]. Anticancer and antiproliferative compound [7, 16]. Apoptosis inducer [8, 23]. Protein kinase D inhibitor. Does not inhibit PKC [9]. Autophagy inducer [10, 23]. Potent SIRT1 (sirtuin 1) activator [11, 22]. Neuroprotective [12, 18]. Adipogenesis inhibitor [13]. PGC-1alpha activator [14]. Sonic hedgehog (Shh) signaling pathway modulator. Gli1 mRNA expression inhibitor. Downregulates Gli transcriptional activity [17]. Cardioprotective [19, 21]. Anti-diabetic [20]. Senescence modulator. Extends lifespan [22]. Inhibitor of NLRP3 inflammasome activation [24].
- SMILESOC1=CC=C(\C=C\C2=CC(O)=CC(O)=C2)C=C1
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200



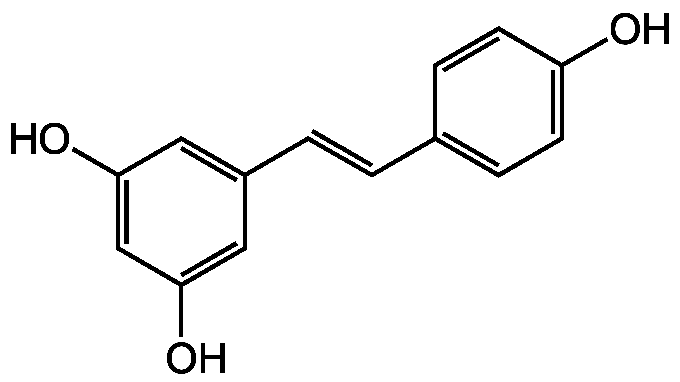
![Resveratrol [501-36-0] [501-36-0]](https://www.targetmol.com/group3/M00/02/B0/CgoaEWY7NVWERG9TAAAAAKsX55k012.png)
![Resveratrol [501-36-0]](https://bpsbioscience.com/media/catalog/product/r/e/resveratrol.png)
