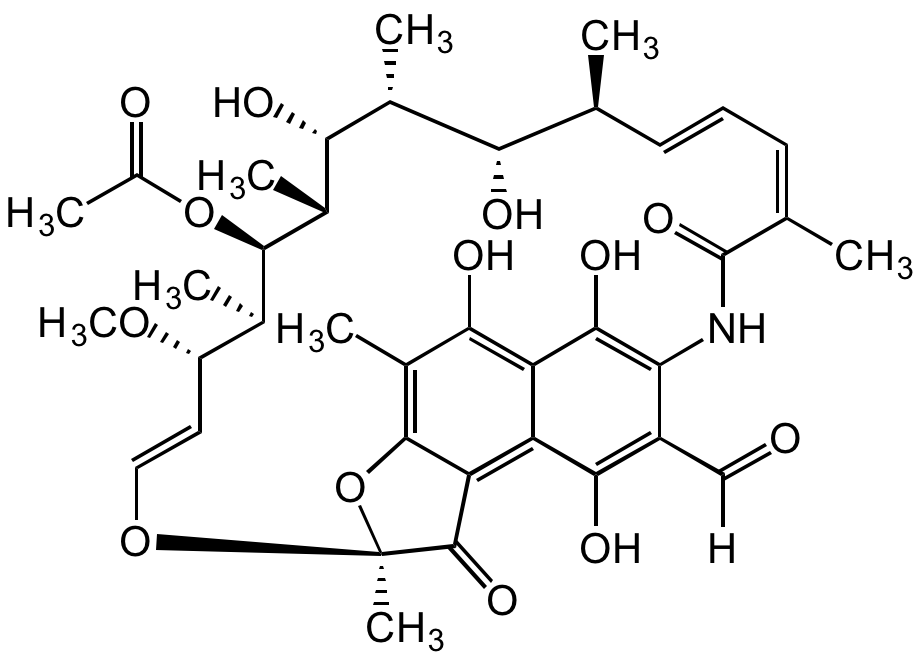
Chemical Structure
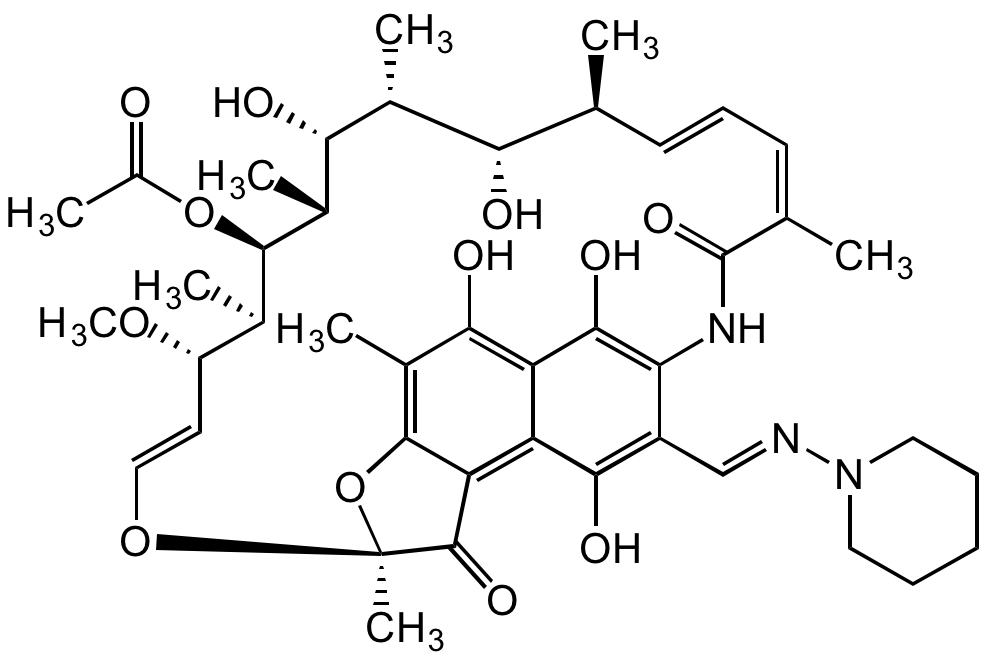
Rifamycin AF [13292-22-3]
AG-CN2-0321
CAS Number13292-22-3
Product group Chemicals
Estimated Purity>95%
Molecular Weight725.8
Overview
- SupplierAdipoGen Life Sciences
- Product NameRifamycin AF [13292-22-3]
- Delivery Days Customer10
- CAS Number13292-22-3
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationWarning
- Molecular FormulaC38H47NO13
- Molecular Weight725.8
- Scientific DescriptionAnsamycin antibiotic. Intermediate of rifampicin. Selective inhibitor of bacterial DNA-dependent RNA polymerase (RNAP). Effective against mycobacteria and therefore used in research of tuberculosis, leprosy and Mycobacterium avium complex (MAC) infections. - Chemical. CAS: 13292-22-3. Formula: C38H47NO13. MW: 725.8. Semisynthetic. Ansamycin antibiotic. Intermediate of rifampicin. Selective inhibitor of bacterial DNA-dependent RNA polymerase (RNAP). Effective against mycobacteria, and are therefore used in research of tuberculosis, leprosy and mycobacterium avium complex (MAC) infections.
- SMILESOC1=C(NC(/C(C)=C\C=C\[C@H](C)[C@H](O)[C@@H](C)[C@H]([C@@H](C)[C@@H]([C@H](C)[C@H](/C=C/O2)OC)OC(C)=O)O)=O)C(C([H])=O)=C(O)C3=C4C(O[C@@]2(C)C4=O)=C(C)C(O)=C31
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12161600