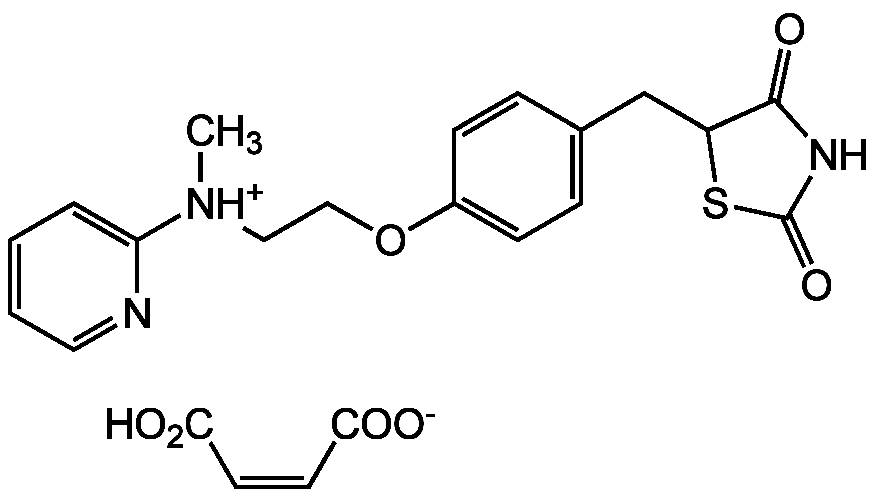
Chemical Structure
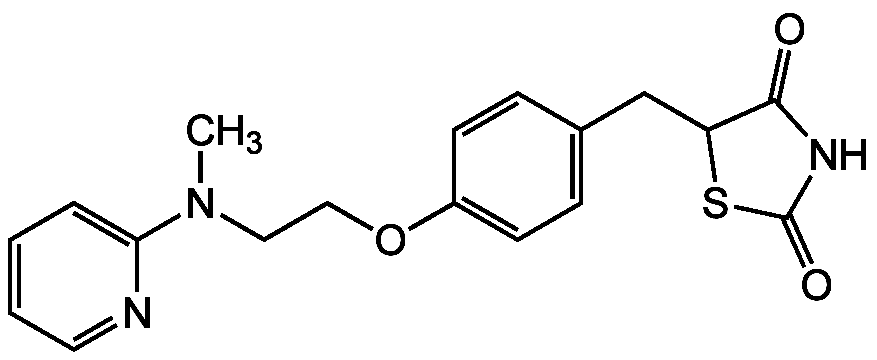
Rosiglitazone . maleate [155141-29-0] [155141-29-0]
AG-CR1-3571
CAS Number155141-29-0
Product group Chemicals
Estimated Purity>98%
Molecular Weight357.4 . 116.1
Overview
- SupplierAdipoGen Life Sciences
- Product NameRosiglitazone . maleate [155141-29-0] [155141-29-0]
- Delivery Days Customer10
- CAS Number155141-29-0
- CertificationResearch Use Only
- Estimated Purity>98%
- Molecular FormulaC18H19N3O3S . C4H4O4
- Molecular Weight357.4 . 116.1
- Scientific DescriptionChemical. CAS: 155141-29-0. Formula: C18H19N3O3S . C4H4O4. MW: 357.4 . 116.1. Same activities as rosaglitazone (Prod. No. AG-CR1-3570) but different formulation. Antidiabetic, hypoglycemic agent. Potent and selective peroxisome proliferator-activated receptor gamma (PPAR-gamma) agonist. Potent insulin sensitizing agent binding to the PPAR receptors in fat cells and making the cells more responsive to insulin. Ameliorates insulin resistance. Improves blood pressure and vascular function. Enhances proliferation of endogenous neural progenitor cells (NPCs). Anti-inflammatory compound. Has controversial therapeutic effects on the cardiovascular system. Promotes adipocyte differentiation of mesenchymal stem cells (MSCs). - Same activities as rosaglitazone (Prod. No. AG-CR1-3570) but different formulation. Antidiabetic, hypoglycemic agent [1, 5]. Potent and selective peroxisome proliferator-activated receptor gamma (PPAR-gamma) agonist [2, 3]. Potent insulin sensitizing agent binding to the PPAR receptors in fat cells and making the cells more responsive to insulin. Ameliorates insulin resistance [4-6, 8]. Improves blood pressure and vascular function [7]. Enhances proliferation of endogenous neural progenitor cells (NPCs) [9]. Anti-inflammatory compound [10, 11]. Has controversial therapeutic effects on the cardiovascular system [12]. Promotes adipocyte differentiation of mesenchymal stem cells (MSCs) [13].
- SMILESOC(=O)\C=C/C([O-])=O.C[NH+](CCOC1=CC=C(CC2SC(=O)NC2=O)C=C1)C1=CC=CC=N1
- Storage Instruction2°C to 8°C
- UNSPSC12352200

![Rosiglitazone maleate [155141-29-0]](https://www.targetmol.com/group3/M00/02/21/CgoaEGY7KbmEDya2AAAAACcKzac377.png)
