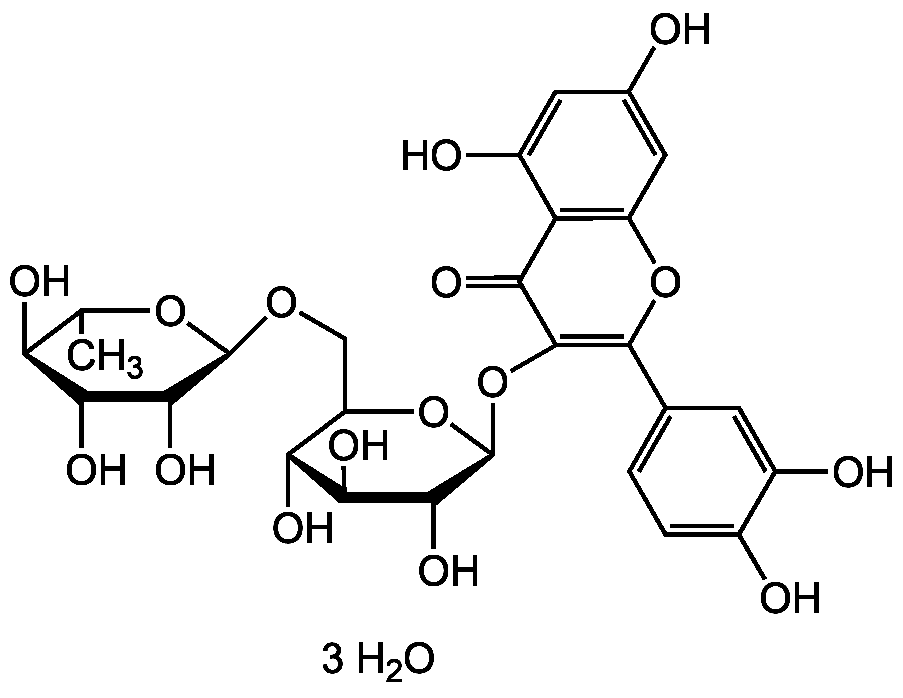
Chemical Structure
Rutin . trihydrate [250249-75-3] [250249-75-3]
AG-CN2-0408
CAS Number250249-75-3
Product group Chemicals
Estimated Purity>95%
Molecular Weight610.5 . 54.0
Overview
- SupplierAdipoGen Life Sciences
- Product NameRutin . trihydrate [250249-75-3] [250249-75-3]
- Delivery Days Customer10
- CAS Number250249-75-3
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationWarning
- Molecular FormulaC27H30O16 . 3H20
- Molecular Weight610.5 . 54.0
- Scientific DescriptionAntioxidant. Free radical scavenger. Nitric oxide (NO) scavenger [1, 5, 6]. Anticancer compound [2, 18]. Antiproliferative [10]. Apoptosis inducer [11]. Reduces induced DNA damage. Protective against carcinogenesis [3]. Hypolipidaemic. Reduces triacylglycerol levels [4]. Anti-inflammatory [5, 15]. Platelet aggregation inhibitor [7]. Anti-hyperglycaemic. Anti-adipogenic [8, 9]. Insulin-mimetic. Activates synthesis of glucose transporter GLUT4 [17]. Neuroprotective [16]. Cardioprotective. Reduces lipid peroxidation [12]. Weak alpha-glucosidase inhibitor [13]. Vasodilatory compound [14]. Brown fat activator [19]. - Chemical. CAS: 250249-75-3. Formula: C27H30O16 . 3H20. MW: 610.5 . 54.0. Isolated from Ruta graveolens. Antioxidant. Free radical scavenger. Nitric oxide (NO) scavenger. Anticancer compound. Antiproliferative. Apoptosis inducer. Reduces induced DNA damage. Protective against carcinogenesis. Hypolipidaemic. Reduces triacylglycerol levels. Anti-inflammatory. Platelet aggregation inhibitor. Anti-hyperglycaemic. Anti-adipogenic. Insulin-mimetic. Activates synthesis of glucose transporter GLUT4. Neuroprotective. Cardioprotective. Reduces lipid peroxidation. Weak alpha-glucosidase inhibitor. Vasodilatory compound.
- SMILESCC1O[C@@H](OCC2O[C@@H](OC3=C(OC4=C(C(O)=CC(O)=C4)C3=O)C3=CC=C(O)C(O)=C3)C(O)[C@H](O)[C@@H]2O)C(O)[C@@H](O)[C@H]1O
- Storage Instruction2°C to 8°C
- UNSPSC12352200

![Rutin trihydrate [250249-75-3] [250249-75-3]](https://www.targetmol.com/group3/M00/02/99/CgoaEGY7ODqEdj9nAAAAAJVIbgU199.png)