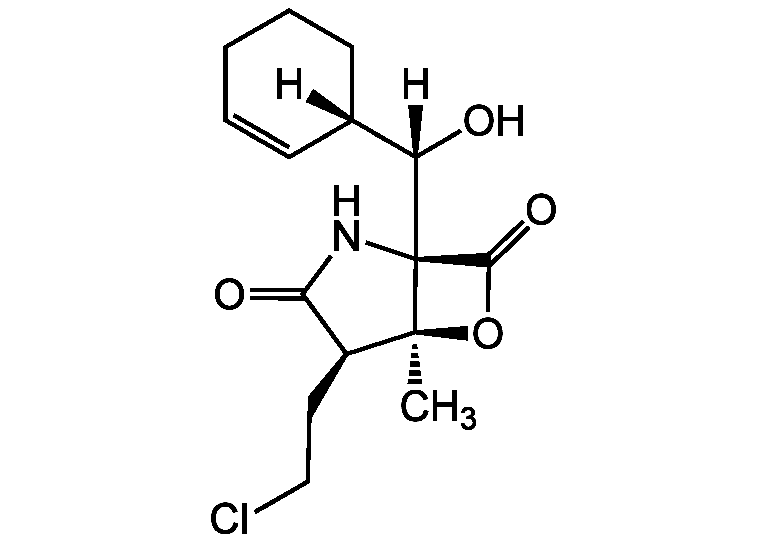
Chemical Structure
Salinosporamide A [437742-34-2] [437742-34-2]
AG-CN2-0444
CAS Number437742-34-2
Product group Chemicals
Estimated Purity>95%
Molecular Weight313.8
Overview
- SupplierAdipoGen Life Sciences
- Product NameSalinosporamide A [437742-34-2] [437742-34-2]
- Delivery Days Customer10
- CAS Number437742-34-2
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationWarning
- Molecular FormulaC15H20ClNO4
- Molecular Weight313.8
- Scientific DescriptionChemical. CAS: 437742-34-2. Formula: C15H20ClNO4. MW: 313.8. Isolated from Salinospora tropica. Potent, irreversible inhibitor of all the 3 proteolytic activities of the mammalian 20S proteasome. beta5 subunit: chymotrypsin-like (EC50 = 3.5nM) beta2 subunit: trypsin-like (EC50 = 28nM) beta1 subunit: caspase-like or peptidyl-glutamyl peptide-hydrolyzing (PGPH) (EC50 = 430nM) Potent anticancer compound. Triggers apoptosis, with distinct proteasome activity and mechanism of action compared to bortezomib (Velcade) (Prod. No. AG-CR1-3602). Most potent suppressor of NF-kappaB activation, compared with bortezomib, MG-132 (Prod. No. AG-CP3-0011), N-acetyl-leucyl-leucyl-norleucinal (ALLN) and lactacystin (Prod. No. AG-CN2-0104). Inhibitor of TNF-alpha, IL-1, IL-6, ICAM-1 and VEGF synthesis. Displays a longer inhibition duration than bortezomib. Potent antileukemic activity against bortezomib-resistant leukemia cells. - Potent, irreversible inhibitor of all the 3 proteolytic activities of the mammalian 20S proteasome. beta5 subunit: chymotrypsin-like (EC50 = 3.5nM) beta2 subunit: trypsin-like (EC50 = 28nM) beta1 subunit: caspase-like or peptidyl-glutamyl peptide-hydrolyzing (PGPH) (EC50 = 430nM) Potent anticancer compound. Triggers apoptosis, with distinct proteasome activity and mechanism of action compared to bortezomib (Velcade) (Prod. No. AG-CR1-3602). Most potent suppressor of NF-kappaB activation, compared with bortezomib, MG-132 (Prod. No. AG-CP3-0011), N-acetyl-leucyl-leucyl-norleucinal (ALLN) and lactacystin (Prod. No. AG-CN2-0104). Inhibitor of TNF-alpha, IL-1, IL-6, ICAM-1 and VEGF synthesis. Displays a longer inhibition duration than bortezomib. Potent antileukemic activity against bortezomib-resistant leukemia cells.
- SMILES[H][C@](O)([C@@]1([H])CCCC=C1)[C@@]12NC(=O)[C@H](CCCl)[C@]1(C)OC2=O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200


![Marizomib [437742-34-2]](https://www.targetmol.com/group3/M00/03/40/CgoaEWY7Rg6EQ6LkAAAAAPQfJFU514.png)