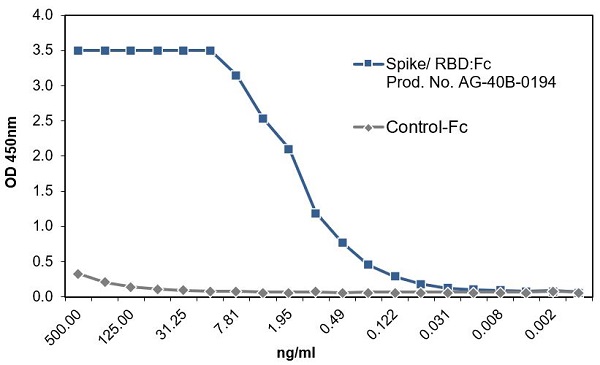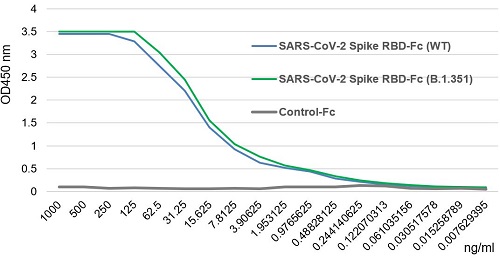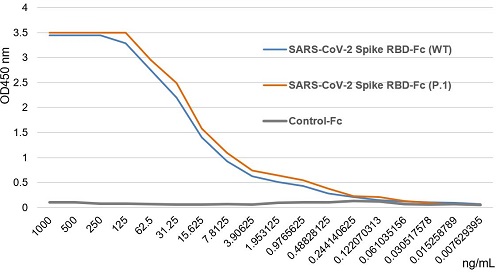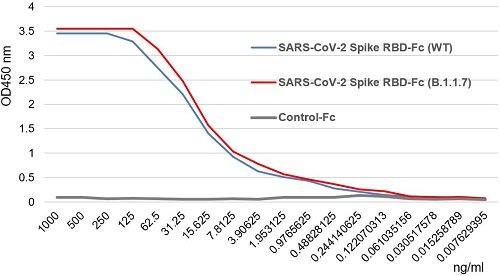
SARS-CoV-2 Spike Protein S1 (RBD):Fc (human) (rec.) (B.1.1.7 Variant, Alpha)
AG-40B-0202
Product group Proteins / Signaling Molecules
Overview
- SupplierAdipoGen Life Sciences
- Product NameSARS-CoV-2 Spike Protein S1 (RBD):Fc (human) (rec.) (B.1.1.7 Variant, Alpha)
- Delivery Days Customer10
- CertificationResearch Use Only
- Concentration1 mg/ml
- Estimated Purity>95%
- Scientific DescriptionRecombinant protein. Receptor-binding domain (RBD) of SARS-CoV-2 Spike protein S1 (aa 319-541) containing the mutation N501Y is fused to the N-terminus of the Fc region of human IgG1. Endotoxin: <0.01EU/microg purified protein (LAL test). Lyophilized. Contains PBS. SARS-CoV-2 shares 79.5% sequence identity with SARS-CoV and is 96.2% identical at the genome level to the bat coronavirus BatCoV RaTG133, suggesting it had originated in bats. The coronaviral genome encodes four major structural proteins: the Spike (S) protein, Nucleocapsid (N) protein, Membrane/Matrix (M) protein and the Envelope (E) protein. The SARS Envelope (E) protein contains a short palindromic transmembrane helical hairpin that seems to deform lipid bilayers, which may explain its role in viral budding and virion envelope morphogenesis. The SARS Membrane/Matrix (M) protein is one of the major structural viral proteins. It is an integral membrane protein involved in the budding of the viral particles and interacts with SARS Spike (S) protein and the Nucleocapsid (N) protein. The N protein contains two domains, both of them bind the virus RNA genome via different mechanisms. The CoV Spike (S) protein assembles as trimer and plays the most important role in viral attachment, fusion and entry. It is composed of a short intracellular tail, a transmembrane anchor and a large ectodomain that consists of a receptor binding S1 subunit (RBD domain) and a membrane-fusing S2 subunit. The S1 subunit contains a receptor binding domain (RBD), which binds to the cell surface receptor angiotensin-converting enzyme 2 (ACE2) present at the surface of epithelial cells. Recently, a more transmissible variant of SARS-CoV-2, called B.1.1.7 (Alpha), was detected in the south of England. This variant carries a mutation in the RBD at the position 501 (N501Y). - SARS-CoV-2 shares 79.5% sequence identity with SARS-CoV and is 96.2% identical at the genome level to the bat coronavirus BatCoV RaTG133, suggesting it had originated in bats. The coronaviral genome encodes four major structural proteins: the Spike (S) protein, Nucleocapsid (N) protein, Membrane/Matrix (M) protein and the Envelope (E) protein. The SARS Envelope (E) protein contains a short palindromic transmembrane helical hairpin that seems to deform lipid bilayers, which may explain its role in viral budding and virion envelope morphogenesis. The SARS Membrane/Matrix (M) protein is one of the major structural viral proteins. It is an integral membrane protein involved in the budding of the viral particles and interacts with SARS Spike (S) protein and the Nucleocapsid (N) protein. The N protein contains two domains, both of them bind the virus RNA genome via different mechanisms. The CoV Spike (S) protein assembles as trimer and plays the most important role in viral attachment, fusion and entry. It is composed of a short intracellular tail, a transmembrane anchor and a large ectodomain that consists of a receptor binding S1 subunit (RBD domain) and a membrane-fusing S2 subunit. The S1 subunit contains a receptor binding domain (RBD), which binds to the cell surface receptor angiotensin-converting enzyme 2 (ACE2) present at the surface of epithelial cells. Recently, a more transmissible variant of SARS-CoV-2, called B.1.1.7 (Alpha), was detected in the south of England. This variant carries a mutation in the RBD at the position 501 (N501Y).
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC41116100