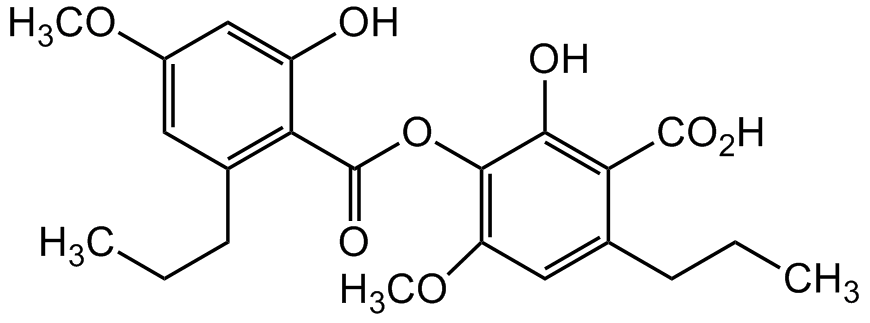
Chemical Structure
Sekikaic acid [607-11-4] [607-11-4]
AG-CN2-0502
CAS Number607-11-4
Product group Chemicals
Estimated Purity>96%
Molecular Weight418.4
Overview
- SupplierAdipoGen Life Sciences
- Product NameSekikaic acid [607-11-4] [607-11-4]
- Delivery Days Customer10
- CAS Number607-11-4
- CertificationResearch Use Only
- Estimated Purity>96%
- Molecular FormulaC22H26O8
- Molecular Weight418.4
- Scientific DescriptionAntioxidant. Reactive oxygen species (ROS) scavenger. Specific ligand of the coactivator CBP/p300, binding to the GACKIX domain. Efficiently inhibits binding of activators at two binding sites. Antibiotic. Potent antibacterial compound. Antifungal on selected fungi species. Antiviral agent. Interferes with the viral replication of selected respiratory syncytial virus. Competitive alpha-glucosidase and non-competitive beta-glucosidase inhibitor. Shows moderate anticancer activity. - Chemical. CAS: 607-11-4. Formula: C22H26O8. MW: 418.4. Isolated from Ramalina sp. Antioxidant. Reactive oxygen species (ROS) scavenger. Specific ligand of the coactivator CBP/p300, binding to the GACKIX domain. Efficiently inhibits binding of activators at two binding sites. Antibiotic. Potent antibacterial compound. Antifungal on selected fungi species. Antiviral agent. Interferes with the viral replication of selected respiratory syncytial virus. Competitive alpha-glucosidase and non-competitive beta-glucosidase inhibitor. Shows moderate anticancer activity.
- SMILESOC1=CC(OC)=CC(CCC)=C1C(OC2=C(OC)C=C(CCC)C(C(O)=O)=C2O)=O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200

![Sekikaic Acid [607-11-4] [607-11-4]](https://www.targetmol.com/group3/M00/02/B2/CgoaEGY7Ox2ESa4MAAAAAOB004M907.png)