Chemical Structure
Siamycin I [164802-68-0] [164802-68-0]
AG-CN2-0146
CAS Number164802-68-0
Product group Chemicals
Estimated Purity>85%
Molecular Weight2163.5
Overview
- SupplierAdipoGen Life Sciences
- Product NameSiamycin I [164802-68-0] [164802-68-0]
- Delivery Days Customer10
- CAS Number164802-68-0
- CertificationResearch Use Only
- Estimated Purity>85%
- Hazard InformationWarning
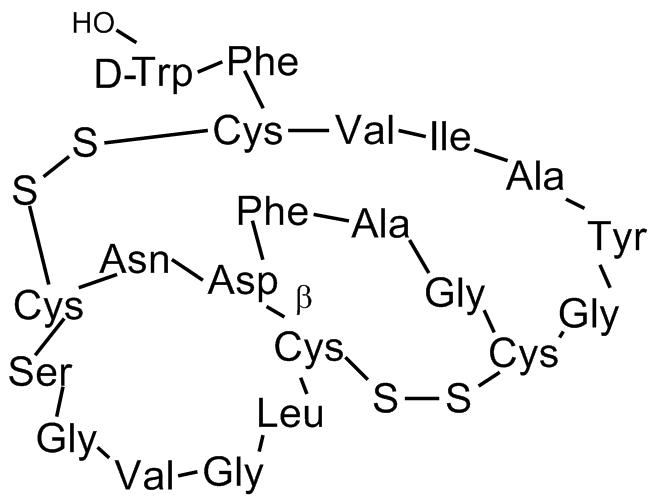
- Molecular FormulaC97H131N23O26S4
- Molecular Weight2163.5
- Scientific DescriptionAntibiotic [1]. Lariat peptide antiviral [1]. Anti-HIV and anti-HSV (Herpes simplex virus) agent [4-6]. Fsr quorum sensing inhibitor [7-9]. Calmodulin-activated myosin light chain kinase (MLCK) inhibitor [2, 3]. - Chemical. CAS: 164802-68-0. Formula: C97H131N23O26S4. MW: 2163.5. Isolated from Streptomyces sp. Antibiotic. Lariat peptide antiviral. Anti-HIV and anti-HSV (Herpes simplex virus) agent. Fsr quorum sensing inhibitor. Calmodulin-activated myosin light chain kinase (MLCK) inhibitor.
- SMILES[H]OC(=O)[C@@]([H])(N([H])C(=O)[C@@]([H])(N([H])C(=O)[C@@]1([H])N([H])C(=O)[C@@]([H])(N([H])C(=O)[C@@]([H])(N([H])C(=O)[C@@]([H])(N([H])C(=O)[C@@]([H])(N([H])C(=O)C([H])([H])N([H])C(=O)[C@@]2([H])N([H])C(=O)C([H])([H])N([H])C(=O)[C@@]([H])(N([H])C(=O)[C@@]([H])(N([H])C(=O)[C@@]3([H])N([H])C(=O)[C@@]([H])(N([H])C(=O)[C@@]([H])(N([H])C(=O)[C@@]([H])(N([H])C(=O)C([H])([H])N([H])C(=O)[C@@]([H])(N([H])C(=O)C([H])([H])N([H])C(=O)[C@@]([H])(N([H])C(=O)[C@@]([H])(N([H])C(=O)C3([H])[H])C([H])([H])SSC2([H])[H])C([H])([H])C([H])(C([H])([H])[H])C([H])([H])[H])C([H])(C([H])([H])[H])C([H])([H])[H])C([H])([H])O[H])C([H])([H])SSC1([H])[H])C([H])([H])C(=O)N([H])[H])C([H])([H])C1=C([H])C([H])=C([H])C([H])=C1[H])C([H])([H])[H])C([H])([H])C1=C([H])C([H])=C(O[H])C([H])=C1[H])C([H])([H])[H])[C@@]([H])(C([H])([H])[H])C([H])([H])C([H])([H])[H])C([H])(C([H])([H])[H])C([H])([H])[H])C([H])([H])C1=C([H])C([H])=C([H])C([H])=C1[H])C([H])([H])C1=C([H])N([H])C2=C([H])C([H])=C([H])C([H])=C12
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200

![Siamycin I [164802-68-0]](https://www.targetmol.com/group3/M00/02/E4/CgoaEWY7O3-EfbsvAAAAAECJh7o745.png)