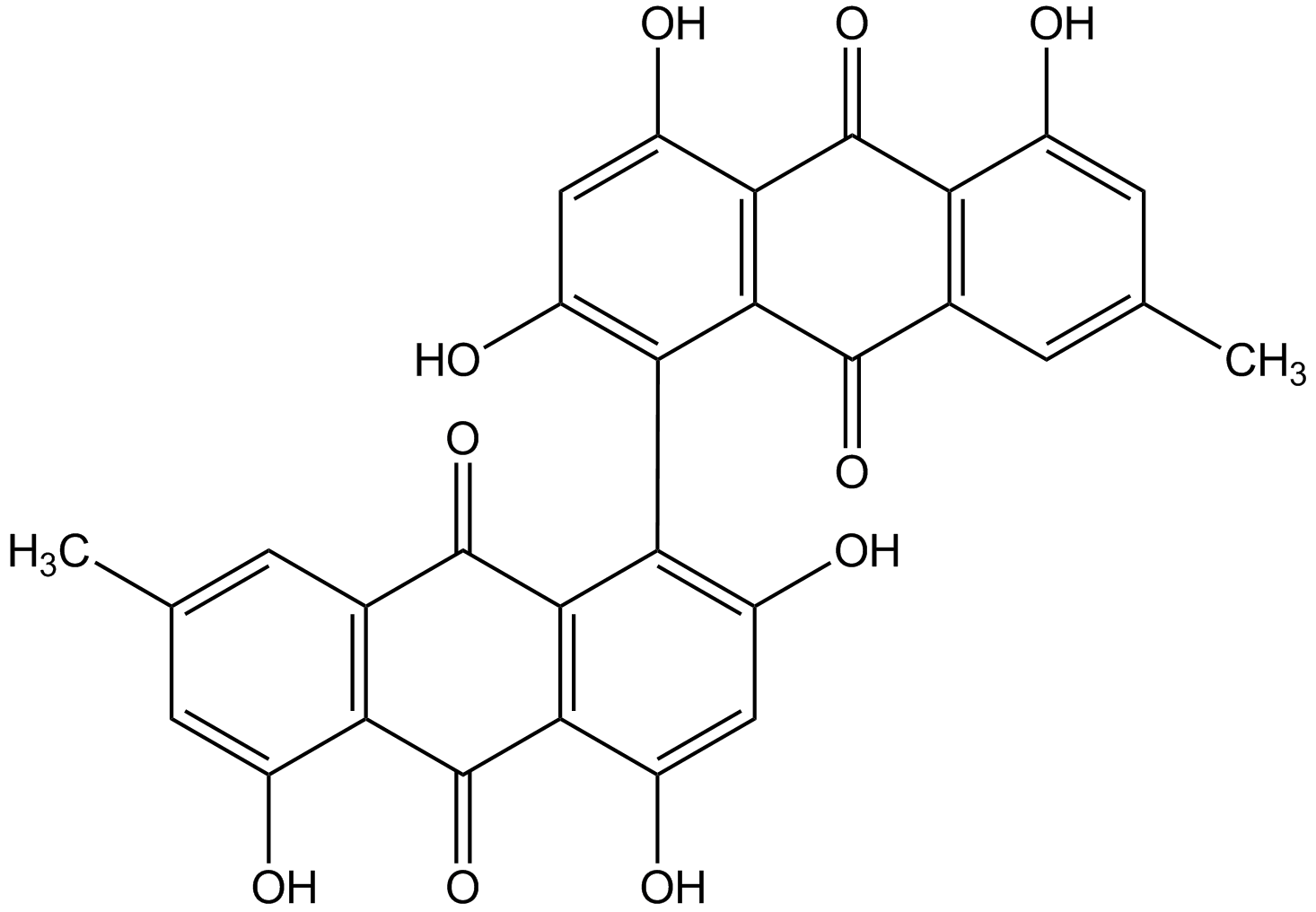
Chemical Structure
Skyrin [602-06-2] [602-06-2]
AG-CN2-0001
CAS Number602-06-2
Product group Chemicals
Estimated Purity>97%
Molecular Weight538.5
Overview
- SupplierAdipoGen Life Sciences
- Product NameSkyrin [602-06-2] [602-06-2]
- Delivery Days Customer10
- CAS Number602-06-2
- CertificationResearch Use Only
- Estimated Purity>97%
- Molecular FormulaC30H18O10
- Molecular Weight538.5
- Scientific DescriptionChemical. CAS: 602-06-2. Formula: C30H18O10. MW: 538.5. Non-peptidic anti-diabetic agent. Receptor-selective glucagon antagonist. Free radical species ( OH, R ) and singlet oxygen (1O2) scavenger. Mycotoxin. Cytotoxic. Antioxidant. Inhibitor of botulinum neurotoxin serotype A (BoNTA). Anti-MRSA compound. Antibiotic. - Non-peptidic anti-diabetic agent [1]. Receptor-selective glucagon antagonist [1]. Free radical species ( OH, R ) and singlet oxygen (1O2) scavenger [2]. Mycotoxin [3, 4]. Cytotoxic [3, 4]. Antioxidant [2]. Inhibitor of botulinum neurotoxin serotype A (BoNTA) [5]. Anti-MRSA compound [6]. Antibiotic [7].
- SMILESCC1=CC2=C(C(O)=C1)C(=O)C1=C(C2=O)C(=C(O)C=C1O)C1=C(O)C=C(O)C2=C1C(=O)C1=C(C(O)=CC(C)=C1)C2=O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200

![Skyrin [602-06-2] [602-06-2]](https://www.targetmol.com/group3/M00/02/51/CgoaEGY7L6aES4OCAAAAAH62f-I667.png)