Chemical Structure
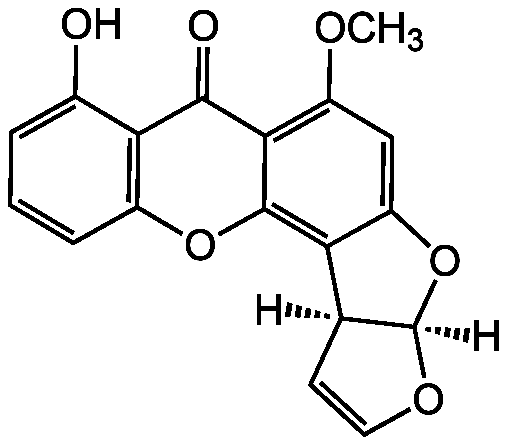
Sterigmatocystin [10048-13-2] [10048-13-2]
BVT-0171
CAS Number10048-13-2
Product group Chemicals
Estimated Purity>98%
Molecular Weight324.3
Overview
- SupplierBioViotica
- Product NameSterigmatocystin [10048-13-2] [10048-13-2]
- Delivery Days Customer2
- CAS Number10048-13-2
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationDanger,Excepted quantity
- Molecular FormulaC18H12O6
- Molecular Weight324.3
- Scientific DescriptionChemical. CAS: 10048-13-2. Formula: C18H12O6. MW: 324.3. Isolated from Aspergillus sp. (strain WDMH51). Intermediate of the biosynthetic pathway to aflatoxin B1. Mycotoxin. DNA synthesis inhibitor. Anticancer compound. Cytotoxic, carcinogenic and mutagenic. Acyl-CoA:cholesterol acyltransferase 2 (ACAT2) inhibitor. Shown to induce apoptosis in human peripheral lymphocytes and necrosis in rat liver. Induces sister chromatid exchanges in murine bone marrow cells. - Intermediate of the biosynthetic pathway to aflatoxin B1. Mycotoxin. DNA synthesis inhibitor. Anticancer compound. Cytotoxic, carcinogenic and mutagenic. Acyl-CoA:cholesterol acyltransferase 2 (ACAT2) inhibitor. Shown to induce apoptosis in human peripheral lymphocytes and necrosis in rat liver. Induces sister chromatid exchanges in murine bone marrow cells.
- SMILES[H][C@]12OC=C[C@@]1([H])C1=C3OC4=C(C(O)=CC=C4)C(=O)C3=C(OC)C=C1O2
- Storage Instruction-20°C,2°C to 8°C
- UN NumberUN 3462
- UNSPSC12352200

![Sterigmatocystine [10048-13-2] [10048-13-2]](https://www.targetmol.com/group3/M00/36/EB/CgoaEGayRYKEDFnQAAAAAGH0SFM491.png)