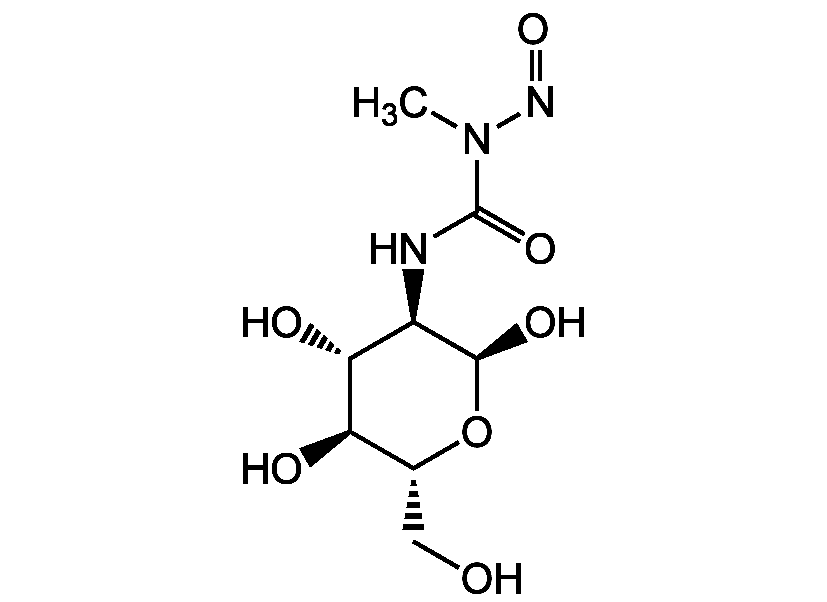
Chemical Structure
Streptozotocin [18883-66-4] [18883-66-4]
AG-CN2-0046
CAS Number18883-66-4
Product group Chemicals
Estimated Purity>98%
Molecular Weight265.2
Overview
- SupplierAdipoGen Life Sciences
- Product NameStreptozotocin [18883-66-4] [18883-66-4]
- Delivery Days Customer10
- CAS Number18883-66-4
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationDanger
- Molecular FormulaC8H15N3O7
- Molecular Weight265.2
- Scientific DescriptionAntibiotic [1]. Diabetogenic. Diabetes inducer. Induces diabetes mellitus in animal models through its toxic effects on pancreatic beta-cells [2, 5, 13, 14]. Mutagenic [3, 10]. Potent alkylating agent. Potent DNA methylating agent [4, 10]. Nitric oxide (NO) donor. Vasorelaxant [6]. Cytotoxic to cells that express GLUT2 glucose transporter [7]. O-GlcNAc-selective N-acetyl-beta-D-glucosaminidase (O-GlcNAcase) inhibitor [8]. Genotoxic. Induces DNA damage. Produces DNA strand breaks [9, 10]. Cell death inducer [15]. Antineoplastic. Anti-cancer agent used in chemotherapy [10, 11]. Induces cell cylce arrest at G2 [12]. - Chemical. CAS: 18883-66-4. Formula: C8H15N3O7. MW: 265.2. Antibiotic. Diabetogenic. Diabetes inducer. Induces diabetes mellitus in animal models through its toxic effects on pancreatic beta-cells. Mutagenic. Potent alkylating agent. Potent DNA methylating agent. Nitric oxide (NO) donor. Vasorelaxant. Cytotoxic to cells that express GLUT2 glucose transporter. O-GlcNAc-selective N-acetyl-beta-D-glucosaminidase (O-GlcNAcase) inhibitor. Genotoxic. Induces DNA damage. Produces DNA strand breaks. Cell death inducer. Antineoplastic. Anti-cancer agent used in chemotherapy. Induces cell cylce arrest at G2.
- SMILESCN(N=O)C(=O)N[C@H]1[C@@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200


![Streptozocin (U-9889) [18883-66-4]](https://bpsbioscience.com/media/catalog/product/s/t/streptozocin.png)

![Streptozocin [18883-66-4]](https://www.targetmol.com/group3/M00/37/FB/CgoaEWayVMOEC68fAAAAANUwb5A639.png)