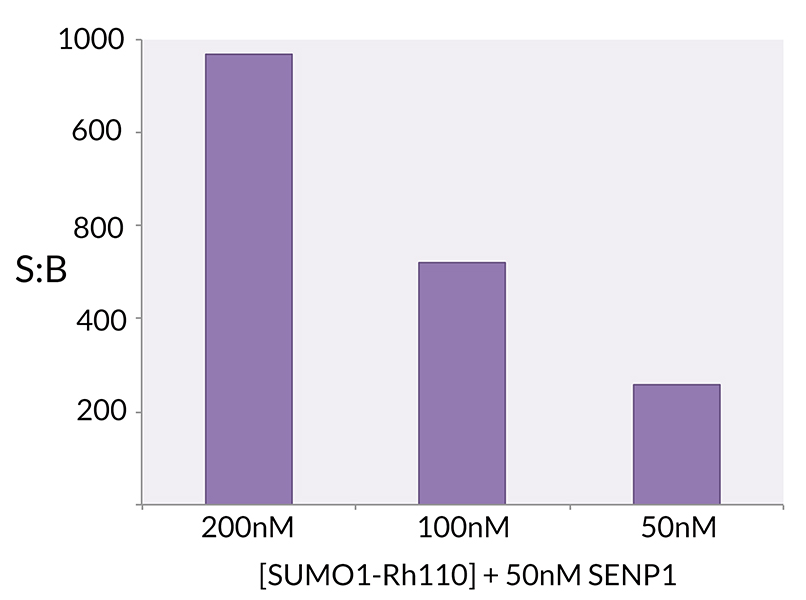
Signal to Background: The signal to background ratio was determined by 100% hydrolysis of 200nM, 100nM, 50nM SUMO1-Rhodamine 110 to liberate the quenched conjugate. Assay Buffer: 50mM HEPES pH 7.5, 1mM TCEP, 0.1mg/ml BSA.
SUMO1 (human) (rec.) (Rhodamine 110)
SBB-PS0028
Protein IDP63165
Product group Proteins / Signaling Molecules
Overview
- SupplierSouth Bay Bio
- Product NameSUMO1 (human) (rec.) (Rhodamine 110)
- Delivery Days Customer2
- CertificationResearch Use Only
- ConjugateTRITC
- Estimated Purity>97%
- Gene ID7341
- Target nameSUMO1
- Target descriptionsmall ubiquitin like modifier 1
- Target synonymsDAP1, GMP1, OFC10, PIC1, SENP2, SMT3, SMT3C, SMT3H3, UBL1, small ubiquitin-related modifier 1, GAP modifying protein 1, SMT3 homolog 3, SMT3 suppressor of mif two 3 homolog 1, sentrin, ubiquitin-homology domain protein PIC1, ubiquitin-like protein SMT3C, ubiquitin-like protein UBL1
- Protein IDP63165
- Protein NameSmall ubiquitin-related modifier 1
- Scientific DescriptionProtein. Human SUMO1 (aa1-97)conjugated at the C-terminus to a quenched Rhodamine 110 dye. Source: E. coli. Formulation: Liquid. In 50mM HEPES pH 7.5, 100mM sodium chloride. Purity: >97% (LCMS). SUMO1 is a ubiquitin-like protein that can be covalently attached to proteins as a monomer or a lysine-linked polymer. Covalent attachment via an isopeptide bond to its substrates requires prior activation by the E1 complex SAE1-SAE2 and linkage to the E2 enzyme UBE2I and can be promoted by E3 ligases such as PIAS1-4, RANBP2 or CBX4. This post-translational modification on lysine residues of proteins plays a crucial role in a number of cellular processes such as nuclear transport, DNA replication and repair, mitosis and signal transduction. This SUMO1 substrate is C-terminally derivatized with a bis-Gly-Rhodamine 110 fluorophore. The bis-Gly-Rh110 is quenched until the amide bond between the C-terminal glycine and the bis-Gly-Rh110 compound is hydrolyzed to mono-Gly-Rhodamine 110. The efficiency of quenching combined with the powerful signal upon hydrolysis yields a reagent with unparalleled signal-to-background. SUMO1-Rh110 can be used to study the deSUMOylating activity of hydrolases SENP1 and SENP2, or other deSUMOylating enzymes. The substrate activity of SUMO1-Rhodamine 110 was determined by measuring the SENP1 catalyzed release of unquenched mono-Gly-Rh110. - SUMO1 is a ubiquitin-like protein that can be covalently attached to proteins as a monomer or a lysine-linked polymer. Covalent attachment via an isopeptide bond to its substrates requires prior activation by the E1 complex SAE1-SAE2 and linkage to the E2 enzyme UBE2I and can be promoted by E3 ligases such as PIAS1-4, RANBP2 or CBX4. This post-translational modification on lysine residues of proteins plays a crucial role in a number of cellular processes such as nuclear transport, DNA replication and repair, mitosis and signal transduction. This SUMO1 substrate is C-terminally derivatized with a bis-Gly-Rhodamine 110 fluorophore. The bis-Gly-Rh110 is quenched until the amide bond between the C-terminal glycine and the bis-Gly-Rh110 compound is hydrolyzed to mono-Gly-Rhodamine 110. The efficiency of quenching combined with the powerful signal upon hydrolysis yields a reagent with unparalleled signal-to-background. SUMO1-Rh110 can be used to study the deSUMOylating activity of hydrolases SENP1 and SENP2, or other deSUMOylating enzymes. The substrate activity of SUMO1-Rhodamine 110 was determined by measuring the SENP1 catalyzed release of unquenched mono-Gly-Rh110.
- Storage Instruction-80°C
- UNSPSC41116100
- SpeciesHuman