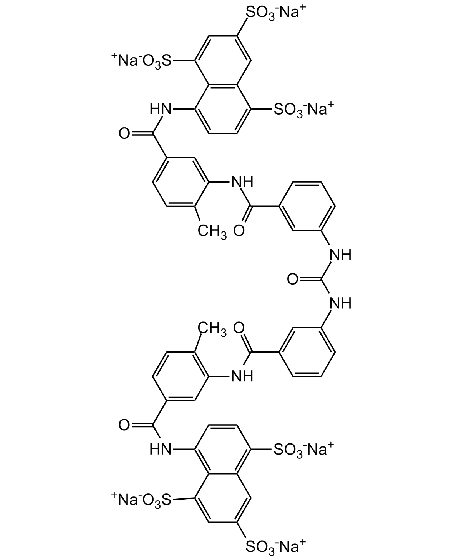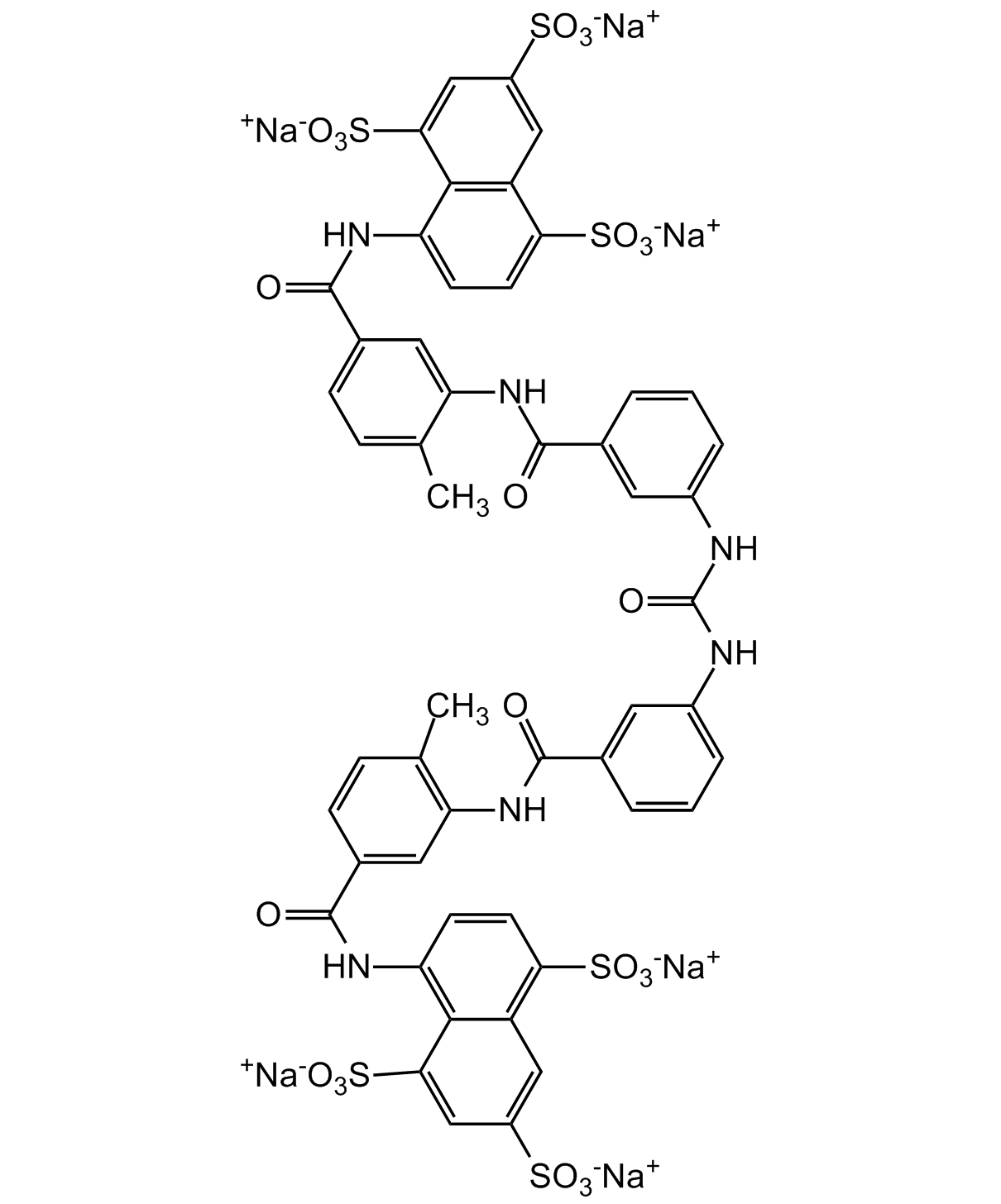
Chemical Structure
Suramin . hexasodium salt [129-46-4] [129-46-4]
AG-CR1-3575
CAS Number129-46-4
Product group Chemicals
Estimated Purity>98%
Molecular Weight1291.2 . 137.9
Overview
- SupplierAdipoGen Life Sciences
- Product NameSuramin . hexasodium salt [129-46-4] [129-46-4]
- Delivery Days Customer10
- CAS Number129-46-4
- CertificationResearch Use Only
- Estimated Purity>98%
- Molecular FormulaC51H34N6O23S6 . 6Na
- Molecular Weight1291.2 . 137.9
- Scientific DescriptionChemical. CAS: 129-46-4. Formula: C51H34N6O23S6 . 6Na. MW: 1291.2 . 137.9. Synthetic. Potent ATPase inhibitor. Potent competitive inhibitor of reverse transcriptase. Shows anti-HIV activity. Anticancer compound. Protein kinase C (PKC) inhibitor. Potent inhibitor of melanoma heparanase and tumor cell metastasis. Non-specific growth factors inhibitor (including PDGF, EGF, aFGF and bFGF). TGF-beta1 inhibitor. Topoisomerase I and II inhibitor. Interleukin-1 (IL-1) inhibitor. Interleukin-4 (IL-4) inhibitor. G protein inhibitor. P2X and P2Y purinergic receptor antagonist. Antiangiogenic. Potent VEGF inhibitor. Telomerase inhibitor. Shows adjuvant properties. Regulates ryanodine receptor. Direct adenylyl cyclase inhibitor. Protein synthesis inhibitor. SIRT1 (sirtuin 1) and SIRT5 (sirtuin 5) inhibitor. Immunosuppressive. Antifibrotic agent. Antiparasitic. Antiprotozoal. Athelmintic. Cullin-RING E3 ubiquitin ligase inhibitor. - Potent ATPase inhibitor [1]. Potent competitive inhibitor of reverse transcriptase. Shows anti-HIV activity [2, 3]. Anticancer compound [4, 5, 15]. Protein kinase C (PKC) inhibitor [4]. Potent inhibitor of melanoma heparanase and tumor cell metastasis [6]. Non-specific growth factors inhibitor (including PDGF, EGF, aFGF and bFGF) [7, 16]. TGF-beta1 inhibitor [8]. Topoisomerase I and II inhibitor [9]. Interleukin-1 (IL-1) inhibitor [10]. Interleukin-4 (IL-4) inhibitor [11]. G protein inhibitor [12]. P2X and P2Y purinergic receptor antagonist [13]. Antiangiogenic. Potent VEGF inhibitor [14, 15]. Telomerase inhibitor [17]. Shows adjuvant properties [18]. Regulates ryanodine receptor [19]. Direct adenylyl cyclase inhibitor [20]. Protein synthesis inhibitor [21]. SIRT1 (sirtuin 1) and SIRT5 (sirtuin 5) inhibitor [22, 23]. Immunosuppressive [24]. Antifibrotic agent [25]. Antiparasitic. Antiprotozoal. Athelmintic [26]. Cullin-RING E3 ubiquitin ligase inhibitor [27]. Inhibitor of the STING pathway via the inhibition of cGAMP synthase (cGAS) enzymatic activity. Inhibits SARS-CoV-2 infection in cell culture by blocking early steps (binding/fusion) of the replication cycle. Potentially binds and inhibits nsp12 of SARS-CoV-2, binding to motifs harbouring the RNA-dependent RNA polymerases (RdRps) activity.
- SMILES[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].CC1=CC=C(C=C1NC(=O)C1=CC(NC(=O)NC2=CC=CC(=C2)C(=O)NC2=C(C)C=CC(=C2)C(=O)NC2=C3C(C=C(C=C3S([O-])(=O)=O)S([O-])(=O)=O)=C(C=C2)S([O-])(=O)=O)=CC=C1)C(=O)NC1=CC=C(C2=C1C(=CC(=C2)S([O-])(=O)=O)S([O-])(=O)=O)S([O-])(=O)=O
- Storage Instruction2°C to 8°C
- UNSPSC12352200


![Suramin Sodium Salt [129-46-4]](https://www.targetmol.com/group3/M00/02/B3/CgoaEGY7OzyESRPrAAAAAPMCEdI167.png)