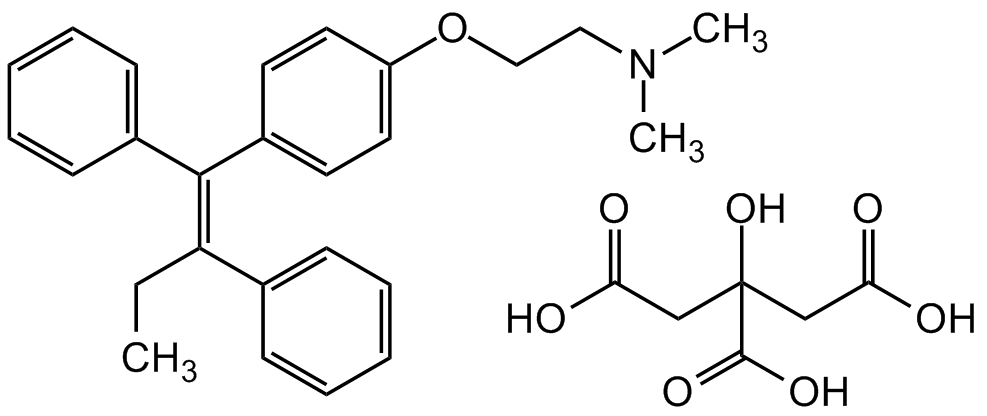
Chemical Structure
Tamoxifen citrate salt [54965-24-1] [54965-24-1]
CDX-T0201
CAS Number54965-24-1
Product group Chemicals
Estimated Purity>98%
Molecular Weight563.64
Overview
- SupplierChemodex
- Product NameTamoxifen citrate salt [54965-24-1] [54965-24-1]
- Delivery Days Customer2
- CAS Number54965-24-1
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationWarning
- Molecular FormulaC26H29NO . C6H8O7
- Molecular Weight563.64
- Scientific DescriptionChemical. CAS: 54965-24-1. Formula: C26H29NO . C6H8O7. MW: 563.64. Synthetic. Selective estrogen receptor modulator (SERM) used as adjuvant therapy for estrogen-dependent breast cancer. Antagonist of ER action in breast tissue and breast cancer cells and ER agonist in bone, uterus, and the cardiovasculature system. This prodrug is metabolized by cytochrome P450 (CYP450) enzymes to the active metabolites N-desmethyl-TMX, 4-hydroxy-N-desmethyl-TMX (endoxifen) and 4-hydroxy-TMX (afimoxifene). N,N-Didesmethyl-4-hydroxytamoxifen (norendoxifen), another active metabolite has been found to act as a potent competitive aromatase inhibitor and may also be involved in its antiestrogenic activity. Binds microsomal antiestrogen binding sites, altering cholesterol esterification at therapeutic doses and impacting breast cancer cell differentiation, apoptosis, and autophagy. Protein kinase C (PKC) inhibitor and anti-angiogenetic factor. - Selective estrogen receptor modulator (SERM) used as adjuvant therapy for estrogen-dependent breast cancer. Antagonist of ER action in breast tissue and breast cancer cells and ER agonist in bone, uterus, and the cardiovasculature system. This prodrug is metabolized by cytochrome P450 (CYP450) enzymes to the active metabolites N-desmethyl-TMX, 4-hydroxy-N-desmethyl-TMX (endoxifen) and 4-hydroxy-TMX (afimoxifene). N,N-Didesmethyl-4-hydroxytamoxifen (norendoxifen), another active metabolite has been found to act as a potent competitive aromatase inhibitor and may also be involved in its antiestrogenic activity. Binds microsomal antiestrogen binding sites, altering cholesterol esterification at therapeutic doses and impacting breast cancer cell differentiation, apoptosis, and autophagy. Protein kinase C (PKC) inhibitor and anti-angiogenetic factor.
- SMILESCN(CCOC1=CC=C(/C(C2=CC=CC=C2)=C(C3=CC=CC=C3)/CC)C=C1)C.OC(CC(O)(C(O)=O)CC(O)=O)=O
- Storage Instruction2°C to 8°C,RT
- UNSPSC12352200


![Tamoxifen Citrate [54965-24-1] [54965-24-1]](https://www.targetmol.com/group3/M00/02/7F/CgoaEGY7NSaEO6abAAAAAEzYNeM392.png)