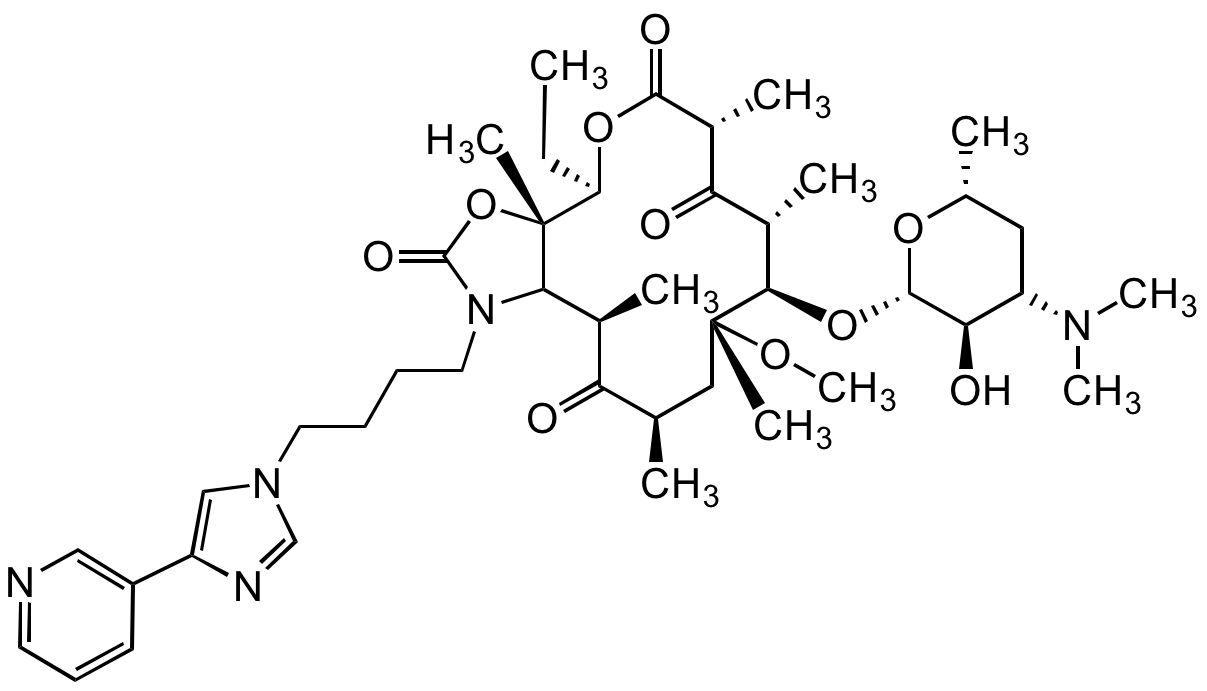
Chemical Structure
Telithromycin [191114-48-4]
BVT-0456
CAS Number191114-48-4
Product group Chemicals
Estimated Purity>98%
Molecular Weight812
Overview
- SupplierBioViotica
- Product NameTelithromycin [191114-48-4]
- Delivery Days Customer2
- CAS Number191114-48-4
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationExcepted quantity,Warning
- Molecular FormulaC43H65N5O10
- Molecular Weight812
- Scientific DescriptionChemical. CAS: 191114-48-4. Formula: C43H65N5O10. MW: 812. Semi-synthetic. Ketolide type macrolide antibiotic. Antibacterial compound used to treat mild to moderate respiratory infections. Protein synthesis inhibitor, by binding to the 50S ribosomal subunit and subsequently blocking the progression of the growing polypeptide chain. Binds to domains II and V of the 23S rRNA of the 50S ribosomal subunit. Has a higher affinity for these ribosomal targets than conventional macrolides due to the additional interactions and increased binding at domain II. Retains activity against Gram-positive cocci in the presence of resistance mediated by methylases (erm genes) that alter the binding site at domain V. May inhibit the formation of ribosomal subunits 50S and 30S. - Ketolide type macrolide antibiotic. Antibacterial compound used to treat mild to moderate respiratory infections. Protein synthesis inhibitor, by binding to the 50S ribosomal subunit and subsequently blocking the progression of the growing polypeptide chain. Binds to domains II and V of the 23S rRNA of the 50S ribosomal subunit. Has a higher affinity for these ribosomal targets than conventional macrolides due to the additional interactions and increased binding at domain II. Retains activity against Gram-positive cocci in the presence of resistance mediated by methylases (erm genes) that alter the binding site at domain V. May inhibit the formation of ribosomal subunits 50S and 30S.
- SMILESCC[C@H]1OC(=O)[C@H](C)C(=O)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)CC([C@H]2O)N(C)C)[C@@](C)(C[C@@H](C)C(=O)[C@H](C)C2N(CCCCN3C=NC(=C3)C3=CN=CC=C3)C(=O)O[C@]12C)OC
- Storage Instruction2°C to 8°C
- UN NumberUN 3077
- UNSPSC12352200


![Telithromycin [191114-48-4]](https://www.targetmol.com/group3/M00/35/88/CgoaEWayI62EHTejAAAAACVk60s874.png)