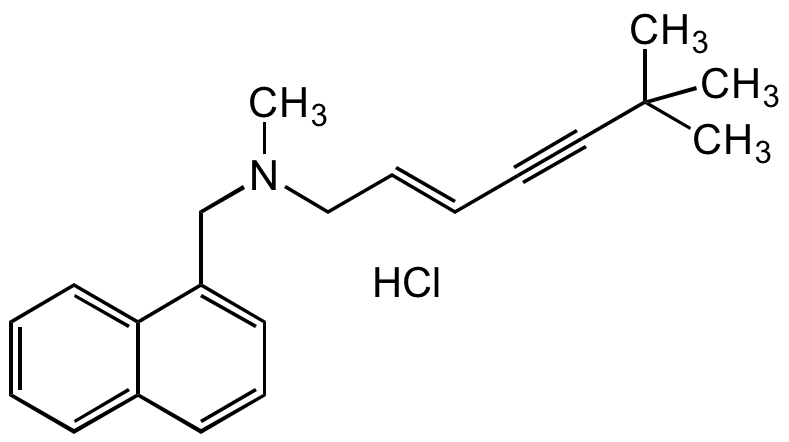
Chemical Structure
Terbinafine hydrochloride [78628-80-5] [78628-80-5]
CDX-T0207
CAS Number78628-80-5
Product group Chemicals
Estimated Purity>98%
Molecular Weight291.4 . 36.5
Overview
- SupplierChemodex
- Product NameTerbinafine hydrochloride [78628-80-5] [78628-80-5]
- Delivery Days Customer2
- CAS Number78628-80-5
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationExcepted quantity,Warning
- Molecular FormulaC21H25N . HCl
- Molecular Weight291.4 . 36.5
- Scientific DescriptionChemical. CAS: 78628-80-5. Formula: C21H25N . HCl. MW: 291.4 . 36.5. Synthetic. Terbinafine is an antifungal and antimycotic compound that is highly active against dermatophytes, mold, other basic fungi, and some strains of yeast. It is clinically used to treat nail and skin infections. Inhibits ergosterol synthesis, essential component of fungal cell membranes. Potent non-competitive inhibitor at the stage of squalene epoxidation (IC50=30nM for C. albicans). Selective activator of the K2P channel TASK3 (pEC50 = 6.2). Exhibits >10-fold selectivity for TASK3 over TREK2, TRESK, THIK1 and TASK2. Also inhibits TWIK1 (pIC50 = 5.69). K2P channels might also be a target for ist antifungal activity. Shown to exhibit at higher concentrations anti-tumor and anti-angiogenic activity by inducing cell cycle arrest, and to display interesting anti-inflammatory and free radical scavenging activities. - Terbinafine is an antifungal and antimycotic compound that is highly active against dermatophytes, mold, other basic fungi, and some strains of yeast. It is clinically used to treat nail and skin infections. Inhibits ergosterol synthesis, essential component of fungal cell membranes. Potent non-competitive inhibitor at the stage of squalene epoxidation (IC50=30nM for C. albicans). Selective activator of the K2P channel TASK3 (pEC50 = 6.2). Exhibits >10-fold selectivity for TASK3 over TREK2, TRESK, THIK1 and TASK2. Also inhibits TWIK1 (pIC50 = 5.69). K2P channels might also be a target for ist antifungal activity. Shown to exhibit at higher concentrations anti-tumor and anti-angiogenic activity by inducing cell cycle arrest, and to display interesting anti-inflammatory and free radical scavenging activities.
- SMILESCN(C/C=C/C#CC(C)(C)C)CC1=CC=CC2=CC=CC=C21.Cl
- Storage Instruction-20°C,2°C to 8°C
- UN Number3077
- UNSPSC12352200


![Terbinafine hydrochloride [78628-80-5] [78628-80-5]](https://www.targetmol.com/group3/M00/02/44/CgoaEGY7LjyEU9PlAAAAAGNXSoQ648.png)