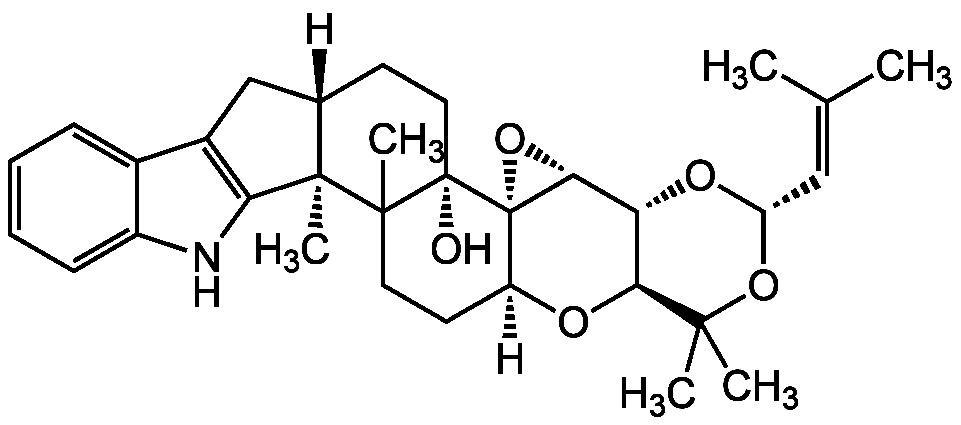
Chemical Structure
Terpendole C [156967-65-6]
AG-CN2-0125
CAS Number156967-65-6
Product group Chemicals
Estimated Purity>95%
Molecular Weight519.7
Overview
- SupplierAdipoGen Life Sciences
- Product NameTerpendole C [156967-65-6]
- Delivery Days Customer10
- CAS Number156967-65-6
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationWarning
- Molecular FormulaC32H41NO5
- Molecular Weight519.7
- Scientific DescriptionAcyl-CoA:cholesterol acyltransferase (ACAT) isozymes ACAT1 and ACAT2 inhibitor [1-3, 6]. Tremorgenic [4, 5]. Cholesteryl ester (CE) synthesis inhibitor [6, 7]. - Chemical. CAS: 156967-65-6. Formula: C32H41NO5. MW: 519.7. Isolated from Albophoma yamanashiensis. Acyl-CoA:cholesterol acyltransferase (ACAT) isozymes ACAT1 and ACAT2 inhibitor. Tremorgenic. Cholesteryl ester (CE) synthesis inhibitor.
- SMILES[H][C@]12CC3=C(NC4=CC=CC=C34)[C@]1(C)C1(C)CC[C@]3([H])O[C@H]4[C@@H](O[C@@H](OC4(C)C)C=C(C)C)[C@H]4O[C@@]34[C@]1(O)CC2
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200

![Terpendole C [156967-65-6]](https://www.targetmol.com/group3/M00/03/78/CgoaEWY7TRGEYpsFAAAAAMz4tI0674.png)