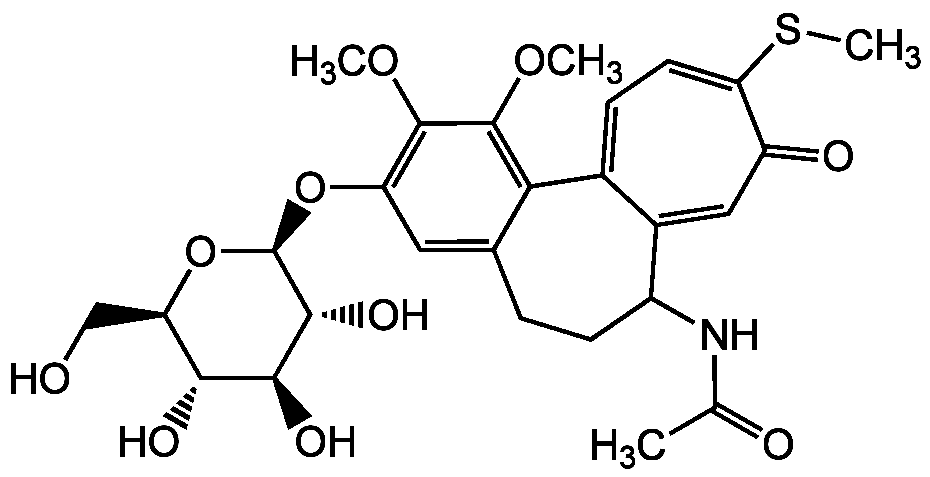
Chemical Structure
Thiocolchicoside [602-41-5] [602-41-5]
AG-CN2-0076
CAS Number602-41-5
Product group Chemicals
Estimated Purity>95%
Molecular Weight563.6
Overview
- SupplierAdipoGen Life Sciences
- Product NameThiocolchicoside [602-41-5] [602-41-5]
- Delivery Days Customer10
- CAS Number602-41-5
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationWarning
- Molecular FormulaC27H33NO10S
- Molecular Weight563.6
- Scientific DescriptionChemical. CAS: 602-41-5. Formula: C27H33NO10S. MW: 563.6. Semisynthetic. Potent competitive gamma-aminobutyric acid type A (GABAA) receptor antagonist and glycine receptor agonist. Weak nicotinic acetylcholine receptor agonist. Muscle relaxant. Anti-inflammatory. Has analgesic properties. Shows strong epileptogenic and convulsant activity. Anticancer compound through inhibition of NF-kappaB and NF-kappaB-regulated gene products Apoptosis inducer. Suppressed osteoclastogenesis induced by RANKL and tumor cells via the NF-kappaB signaling pathway. Therapeutic option for the management of bone metastatic disease. - Potent competitive gamma-aminobutyric acid type A (GABAA) receptor antagonist and glycine receptor agonist. Weak nicotinic acetylcholine receptor agonist. Muscle relaxant. Anti-inflammatory. Has analgesic properties. Shows strong epileptogenic and convulsant activity. Anticancer compound through inhibition of NF-kappaB and NF-kappaB-regulated gene products Apoptosis inducer. Suppressed osteoclastogenesis induced by RANKL and tumor cells via the NF-kappaB signaling pathway. Therapeutic option for the management of bone metastatic disease.
- SMILESCOC1=C(O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)C=C2CCC(NC(C)=O)C3=CC(=O)C(SC)=CC=C3C2=C1OC
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200

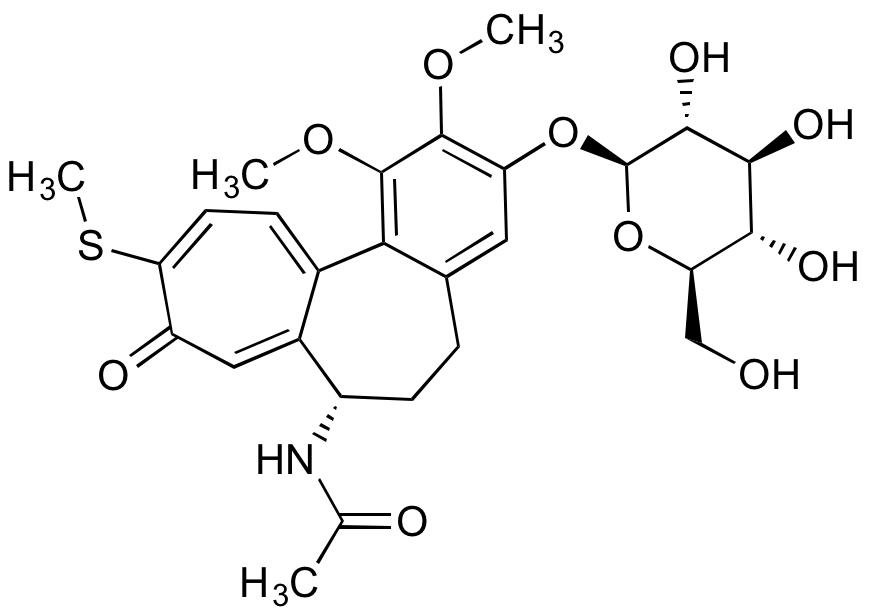
![Thiocolchicoside [602-41-5] [602-41-5]](https://www.targetmol.com/group3/M00/37/49/CgoaEWayStWEVRMjAAAAAICYn3Q078.png)