Chemical Structure
Umifenovir Sterile Solution
IAX-700-106
Estimated Purity>95%
Product group Chemicals
Molecular Weight477.4 . 36.5
Overview
- SupplierInnaxon
- Product NameUmifenovir Sterile Solution
- Delivery Days Customer10
- CertificationResearch Use Only
- Concentration1 mg/ml
- Estimated Purity>95%
- Hazard InformationWarning
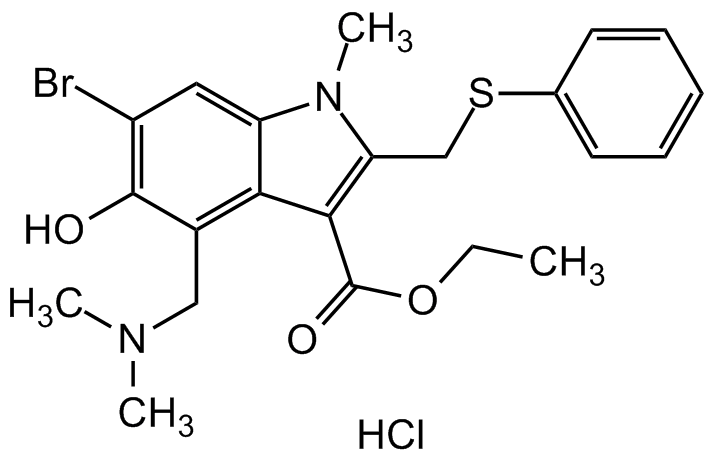
- Molecular FormulaC22H25BrN2O3S . HCl
- Molecular Weight477.4 . 36.5
- Scientific DescriptionChemical. CAS: 131707-23-8. Formula: C22H25BrN2O3S . HCl. MW: 477.4 . 36.5. Umifenovir Lipodisq Sterile Solution is a ready-to-use nano-formulated aqueous solution. Umifenovir (Arbidol) is known to have broad-spectrum anti-viral activity and has earlier been approved in China and Russia for treating influenza, SARS, and Lassa viruses. It has been tested in multiple clinical studies as a candidate for use as an anti-COVID19 therapeutic and has been suggested to act at the entry stage and at the post-entry stages by preventing viral attachment and inhibiting the release of virus particles from intracellular vesicles, respectively. In a recent phase III, clinical study Umifenovir met the primary and secondary endpoint criteria. It has been shown to efficacious, safe and well-tolerated at the tested dosage. Umifenovir Lipodisq is based on a nanoparticle (11-40nm) drug delivery system comprising a discoidal phospholipid bilayer membrane stabilized by a chaperone molecule annulus. Internal properties of the phospholipid membrane support the disposition and stabilization of drug molecule candidates and preserve the native conformation of membrane molecules. The resulting encapsulated actives are rendered water-soluble and specialized for intra-cellular penetration/delivery via endosomal uptake mechanisms. Lipodisq solutions show a good safety profile and are suitable for in vitro and in vivo investigations. - Umifenovir Lipodisq Sterile Solution is a ready-to-use nano-formulated aqueous solution. Umifenovir (Arbidol) is known to have broad-spectrum anti-viral activity and has earlier been approved in China and Russia for treating influenza, SARS, and Lassa viruses. It has been tested in multiple clinical studies as a candidate for use as an anti-COVID19 therapeutic and has been suggested to act at the entry stage and at the post-entry stages by preventing viral attachment and inhibiting the release of virus particles from intracellular vesicles, respectively. In a recent phase III, clinical study Umifenovir met the primary and secondary endpoint criteria. It has been shown to efficacious, safe and well-tolerated at the tested dosage. Umifenovir Lipodisq is based on a nanoparticle (11-40nm) drug delivery system comprising a discoidal phospholipid bilayer membrane stabilized by a chaperone molecule annulus. Internal properties of the phospholipid membrane support the disposition and stabilization of drug molecule candidates and preserve the native conformation of membrane molecules. The resulting encapsulated actives are rendered water-soluble and specialized for intra-cellular penetration/delivery via endosomal uptake mechanisms. Lipodisq solutions show a good safety profile and are suitable for in vitro and in vivo investigations.
- SMILESBrC1=C(O)C(CN(C)C)=C(C(C(OCC)=O)=C(CSC2=CC=CC=C2)N3C)C3=C1.Cl
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
