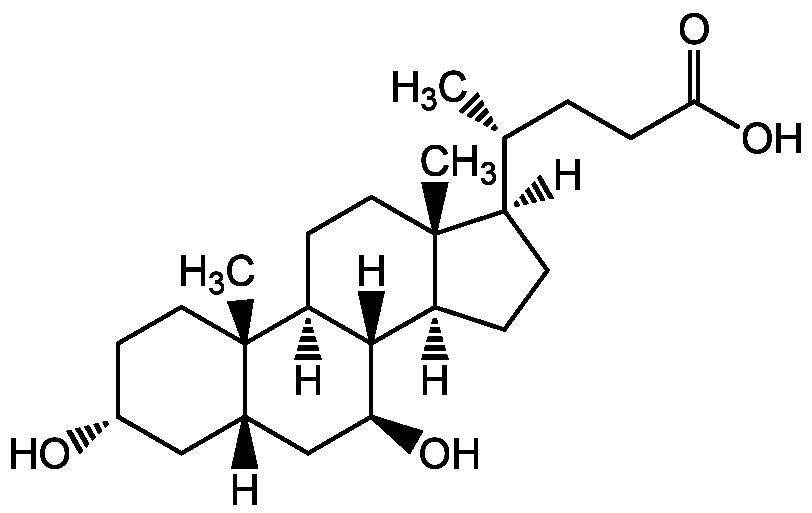
Chemical Structure
Ursodeoxycholic acid [128-13-2] [128-13-2]
AG-CN2-0411
CAS Number128-13-2
Product group Chemicals
Estimated Purity>95%
Molecular Weight392.6
Overview
- SupplierAdipoGen Life Sciences
- Product NameUrsodeoxycholic acid [128-13-2] [128-13-2]
- Delivery Days Customer10
- CAS Number128-13-2
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationWarning
- Molecular FormulaC24H40O4
- Molecular Weight392.6
- Scientific DescriptionChemical. CAS: 128-13-2. Formula: C24H40O4. MW: 392.6. Synthetic. Endogenous hydrophilic bile acid. Antioxidant. Cytoprotective against oxidative stress and cell death. Hepatoprotective at cellular and molecular level, including stabilization of membranes. Protects hepatocytes against bile acid-induced apoptosis. Antiapoptotic and antinecrotic. Targets the mitochondrial function and integrity, reduction of endoplasmatic stress and interactions with survival signals in cAMP, Akt, NF-kappaB, MAPK and PI3K signaling pathways. Modulator and finetuner of the p53-Mdm-2 complex. Chemopreventive against colorectal cancer by countering the tumor-promoting effects of secondary bile acids. Shows also effects on epidermal growth factor receptor (EGFR) signaling and COX-2 expression. Immunomodulator and anti-inflammatory compound. Modifies TLR4 and TLR9 signaling pathways and downregulates the production of proinflammatory tumor necrosis factor-alpha (TNF-alpha). Pregnane X receptor agonist. Neuroprotective. Inhibits neuronal apoptosis. Glucocorticoid Receptor (GR) agonist. Anticholestatic agent. Used to reduce cholesterol absorption and for cholesterol gallstone dissolution. Used to treat primary biliary cirrhosis (PBC). Interferes with the progression of non-alcoholic fatty liver disease (NAFLD)/NASH. Reduces CXCR3 expression. TIMP-1 inducer. ADAM17 inhibitor. - Endogenous hydrophilic bile acid. Antioxidant. Cytoprotective against oxidative stress and cell death. Hepatoprotective at cellular and molecular level, including stabilization of membranes. Protects hepatocytes against bile acid-induced apoptosis. Antiapoptotic and antinecrotic. Targets the mitochondrial function and integrity, reduction of endoplasmatic stress and interactions with survival signals in cAMP, Akt, NF-kappaB, MAPK and PI3K signaling pathways. Modulator and finetuner of the p53-Mdm-2 complex. Chemopreventive against colorectal cancer by countering the tumor-promoting effects of secondary bile acids. Shows also effects on epidermal growth factor receptor (EGFR) signaling and COX-2 expression. Immunomodulator and anti-inflammatory compound. Modifies TLR4 and TLR9 signaling pathways and downregulates the production of proinflammatory tumor necrosis factor-alpha (TNF-alpha). Pregnane X receptor agonist. Neuroprotective. Inhibits neuronal apoptosis. Glucocorticoid Receptor (GR) agonist. Anticholestatic agent. Used to reduce cholesterol absorption and for cholesterol gallstone dissolution. Used to treat primary biliary cirrhosis (PBC). Interferes with the progression of non-alcoholic fatty liver disease (NAFLD)/NASH. Reduces CXCR3 expression [11]. TIMP-1 inducer [12]. ADAM17 inhibitor [12].
- SMILES[H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(O)=O
- Storage Instruction2°C to 8°C
- UNSPSC12352200


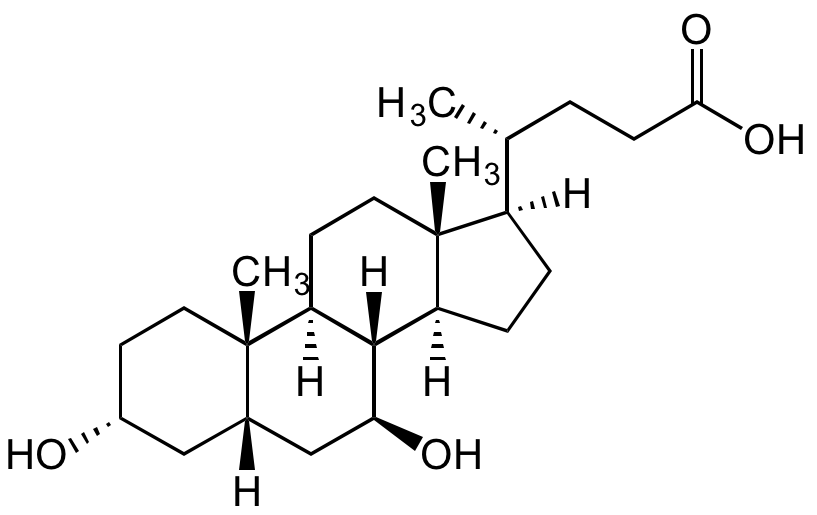
![Ursodeoxycholic acid [128-13-2] [128-13-2]](https://www.targetmol.com/group3/M00/03/45/CgoaEGY7TFWEelIQAAAAAME18RA697.png)