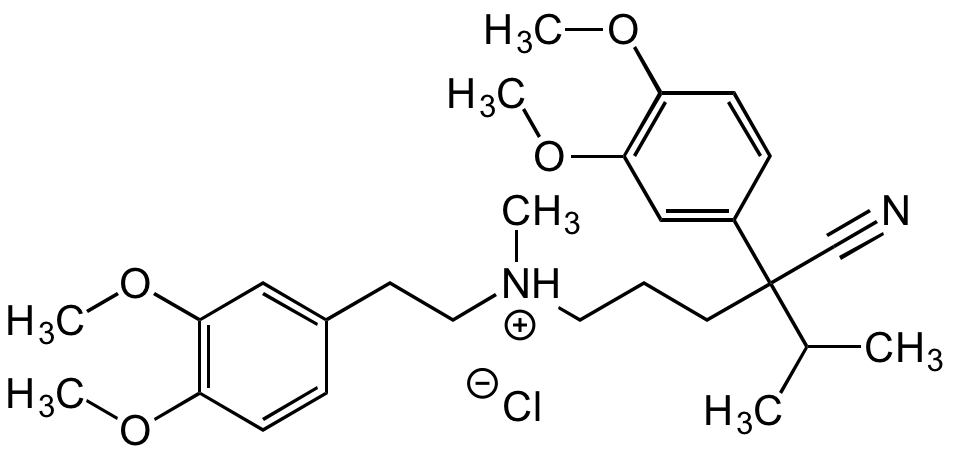
Chemical Structure
(+/-)-Verapamil . hydrochloride (USP Grade) [152-11-4] [152-11-4]
AG-CR1-3627
CAS Number152-11-4
Product group Chemicals
Estimated Purity>99%
Molecular Weight454.6 . 36.5
Overview
- SupplierAdipoGen Life Sciences
- Product Name(+/-)-Verapamil . hydrochloride (USP Grade) [152-11-4] [152-11-4]
- Delivery Days Customer10
- CAS Number152-11-4
- CertificationResearch Use Only
- Estimated Purity>99%
- Hazard InformationDanger,Excepted quantity
- Molecular FormulaC27H38N2O4 . HCl
- Molecular Weight454.6 . 36.5
- Scientific Descriptionalpha1-Adrenergic receptor (alpha-AR) antagonist. Prototypical calcium channel protein inhibitor that blocks the L-type Ca2+ channels in smooth and cardiac muscle cells. Vasodilator known to reduce the renal clearance of digoxin and used to control hypertension, angina, cardiac arrhythmia and vascular headaches. Apoptosis inducer in primary and metastatic colon adenocarcinoma human cell lines in vitro. Inhibitor of drug efflux pump proteins such as Mdr (P-glycoprotein) which are often over-expressed in certain tumor cell lines. Substrate of CYP3A4 and CYP2C6. Used in fluorescent cell sorting for DNA content, as it blocks efflux of a variety of DNA-binding fluorophores such as Hoechst 33342. Anti-diabetic in animal models by limiting TXNIP expression. - Chemical. CAS: 152-11-4. Formula: C27H38N2O4 . HCl. MW: 454.6 . 36.5. Synthetic. alpha1-Adrenergic receptor (alpha-AR) antagonist. Prototypical calcium channel protein inhibitor that blocks the L-type Ca2+ channels in smooth and cardiac muscle cells. Vasodilator known to reduce the renal clearance of digoxin and used to control hypertension, angina, cardiac arrhythmia and vascular headaches. Apoptosis inducer in primary and metastatic colon adenocarcinoma human cell lines in vitro. Inhibitor of drug efflux pump proteins such as Mdr (P-glycoprotein) which are often over-expressed in certain tumor cell lines. Substrate of CYP3A4 and CYP2C6. Used in fluorescent cell sorting for DNA content, as it blocks efflux of a variety of DNA-binding fluorophores such as Hoechst 33342. Anti-diabetic in animal models by limiting TXNIP expression.
- SMILES[Cl-].COC1=C(OC)C=C(CC[NH+](C)CCCC(C#N)(C(C)C)C2=CC(OC)=C(OC)C=C2)C=C1
- Storage Instruction2°C to 8°C
- UN NumberUN 2811
- UNSPSC12352200



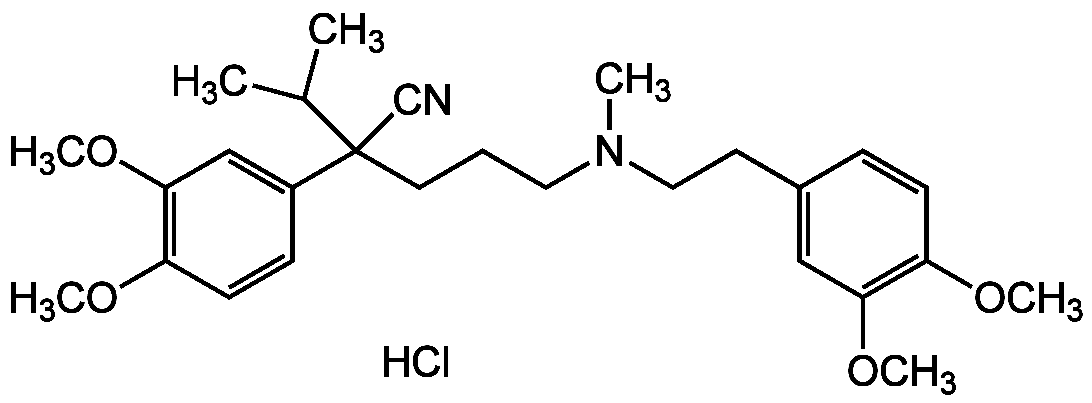
![Verapamil hydrochloride [152-11-4] [152-11-4]](https://www.targetmol.com/group3/M00/02/D8/CgoaEGY7P4iEVGR6AAAAAF8Z0Us126.png)