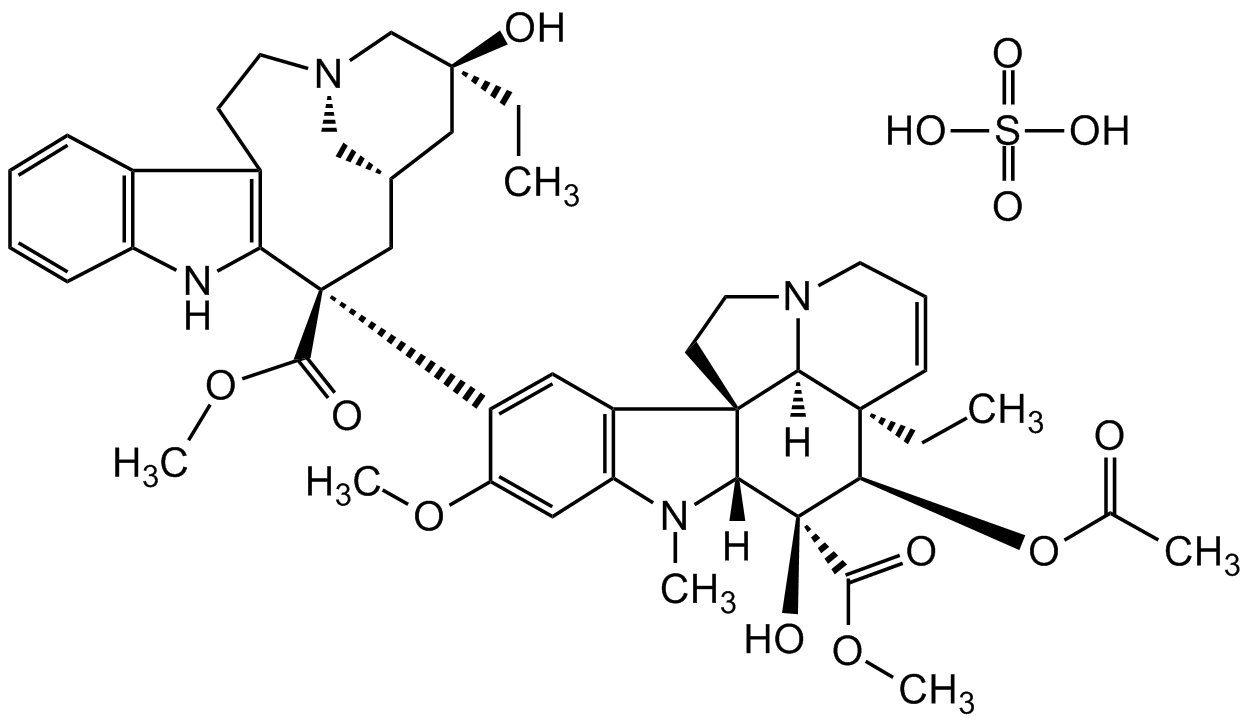
Chemical Structure
Vinblastine sulfate salt [143-67-9] [143-67-9]
CDX-V0013
CAS Number143-67-9
Product group Chemicals
Estimated Purity>97%
Molecular Weight909.05
Overview
- SupplierChemodex
- Product NameVinblastine sulfate salt [143-67-9] [143-67-9]
- Delivery Days Customer2
- CAS Number143-67-9
- CertificationResearch Use Only
- Estimated Purity>97%
- Hazard InformationWarning
- Molecular FormulaC46H58N4O9 . H2SO4
- Molecular Weight909.05
- Scientific DescriptionChemical. CAS: 143-67-9. Formula: C46H58N4O9 . H2SO4. MW: 909.05. Vinblastine sulfate is a vinca alkaloid and a chemical analog of vincristine. It is an anticancer agent with anti-mitotic properties and immunosuppressive activity. Vinblastine sulfate depolymerizes microtubules by binding tubulin and inducing self-association in spiral aggregates. This reaction that appears to be regulated by the C-terminus of beta-tubulin and is enhanced by GDP and GTP. Vinblastine sulfate has been observed to arrest the cell cycle in the G2/M-phase by blocking mitotic spindle formation. Vinblastine sulfate has also been documented to trigger Raf-1 activation and phosphorylation of Bcl-2 family proteins, to induce p53 expression, and to induce apoptosis in several tumor cell lines. Shown to inhibit autophagosome maturation. - Vinblastine sulfate is a vinca alkaloid and a chemical analog of vincristine. It is an anticancer agent with anti-mitotic properties and immunosuppressive activity. Vinblastine sulfate depolymerizes microtubules by binding tubulin and inducing self-association in spiral aggregates. This reaction that appears to be regulated by the C-terminus of beta-tubulin and is enhanced by GDP and GTP. Vinblastine sulfate has been observed to arrest the cell cycle in the G2/M-phase by blocking mitotic spindle formation. Vinblastine sulfate has also been documented to trigger Raf-1 activation and phosphorylation of Bcl-2 family proteins, to induce p53 expression, and to induce apoptosis in several tumor cell lines. Shown to inhibit autophagosome maturation.
- SMILESO=S(O)(O)=O.O[C@@]1(C(OC)=O)[C@]2([H])N(C)C3=C(C=C([C@@](C(OC)=O)(C[C@@H](C[C@]4(CC)O)C[N@@](C4)CC5)C6=C5C(C=CC=C7)=C7N6)C(OC)=C3)[C@]28[C@@]([C@](C=CC9)(CC)[C@H]1OC(C)=O)([H])N9CC8
- Storage Instruction2°C to 8°C,RT
- UNSPSC12352200


![Vinblastine sulfate [143-67-9] [143-67-9]](https://www.targetmol.com/group3/M00/02/7D/CgoaEWY7L1GEUFkDAAAAAHLWB_M005.png)