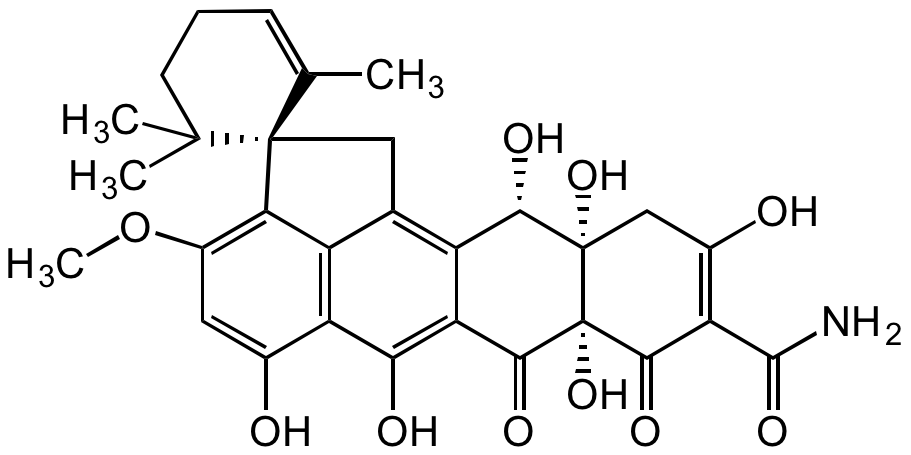
Chemical Structure
Viridicatumtoxin [39277-41-3] [39277-41-3]
AG-CN2-0172
CAS Number39277-41-3
Product group Chemicals
Estimated Purity>95%
Molecular Weight565.6
Overview
- SupplierAdipoGen Life Sciences
- Product NameViridicatumtoxin [39277-41-3] [39277-41-3]
- Delivery Days Customer10
- CAS Number39277-41-3
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationWarning
- Molecular FormulaC30H31NO10
- Molecular Weight565.6
- Scientific DescriptionChemical. CAS: 39277-41-3. Formula: C30H31NO10. MW: 565.6. Isolated from Penicillium brasilianum. Mycotoxin. Tetracycline antibiotic. Potent antibacterial agent with activity against several strains of Staphylococcus aureus, including Methicillin-resistant S. aureus (MRSA) and quinolone-resistant S. aureus (QRSA). Inhibits bacterial undecaprenyl pyrophosphate (UPP) synthase activity. Modest antitumor agent against selected cancer cell lines (IC50~1microM). - Mycotoxin. Tetracycline antibiotic. Potent antibacterial agent with activity against several strains of Staphylococcus aureus, including Methicillin-resistant S. aureus (MRSA) and quinolone-resistant S. aureus (QRSA). Inhibits bacterial undecaprenyl pyrophosphate (UPP) synthase activity. Modest antitumor agent against selected cancer cell lines (IC50~1microM).
- SMILESOC(C[C@]1(O)[C@@H](O)C2=C(C[C@]34C(C)=CCCC4(C)C)C(C3=C(OC)C=C5O)=C5C(O)=C2C6=O)=C(C(N)=O)C([C@]16O)=O
- Storage Instruction-20°C,2°C to 8°C
- UN Number2811
- UNSPSC12352200
