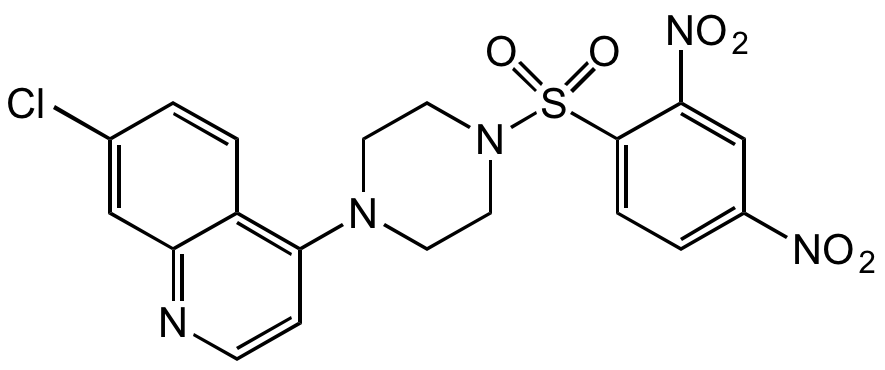
Chemical Structure
VR23 [Proteasome Inhibitor] [1624602-30-7]
AG-CR1-3676
CAS Number1624602-30-7
Product group Chemicals
Estimated Purity>98%
Molecular Weight477.9
Overview
- SupplierAdipoGen Life Sciences
- Product NameVR23 [Proteasome Inhibitor] [1624602-30-7]
- Delivery Days Customer10
- CAS Number1624602-30-7
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationWarning
- Molecular FormulaC19H16ClN5O6S
- Molecular Weight477.9
- Scientific DescriptionChemical. CAS: 1624602-30-7. Formula: C19H16ClN5O6S. MW: 477.9. Synthetic. Potent selective and reversible chloroquine derivative 20S proteasome inhibitor (all proteolytic subunits). Targets the trypsin-like beta2 subunit of the constitutive 20S proteasome (IC50=1nM). Cross-reacts and inhibits the chymotrypsin-like beta5 subunit (IC50=50-100nM) and the caspase-like/peptidyl-glutamyl peptide-hydrolyzing (PGPH) beta1 subunit (IC50=3microM). Anticancer compound effective in cell-based assays and against multiple myeloma and metastatic breast cancer in vivo. In vitro, induces cell cycle arrest and apoptosis in human cancer cell lines including multiple myeloma, as well as in bortezomib-resistent multiple myeloma cells, but with minimal effects on non-cancerous cells. - Potent selective and reversible chloroquine derivative 20S proteasome inhibitor (all proteolytic subunits). Targets the trypsin-like beta2 subunit of the constitutive 20S proteasome (IC50=1nM). Cross-reacts and inhibits the chymotrypsin-like beta5 subunit (IC50=50-100nM) and the caspase-like/peptidyl-glutamyl peptide-hydrolyzing (PGPH) beta1 subunit (IC50=3microM). Anticancer compound effective in cell-based assays and against multiple myeloma and metastatic breast cancer in vivo. In vitro, induces cell cycle arrest and apoptosis in human cancer cell lines including multiple myeloma, as well as in bortezomib-resistent multiple myeloma cells, but with minimal effects on non-cancerous cells.
- SMILESO=S(N1CCN(C2=C(C=CC(Cl)=C3)C3=NC=C2)CC1)(C4=CC=C([N+]([O-])=O)C=C4[N+]([O-])=O)=O
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12161509


![VR23 [1624602-30-7] [1624602-30-7]](https://www.targetmol.com/group3/M00/35/78/CgoaEWayIOmELU_JAAAAAMBdF1U081.png)