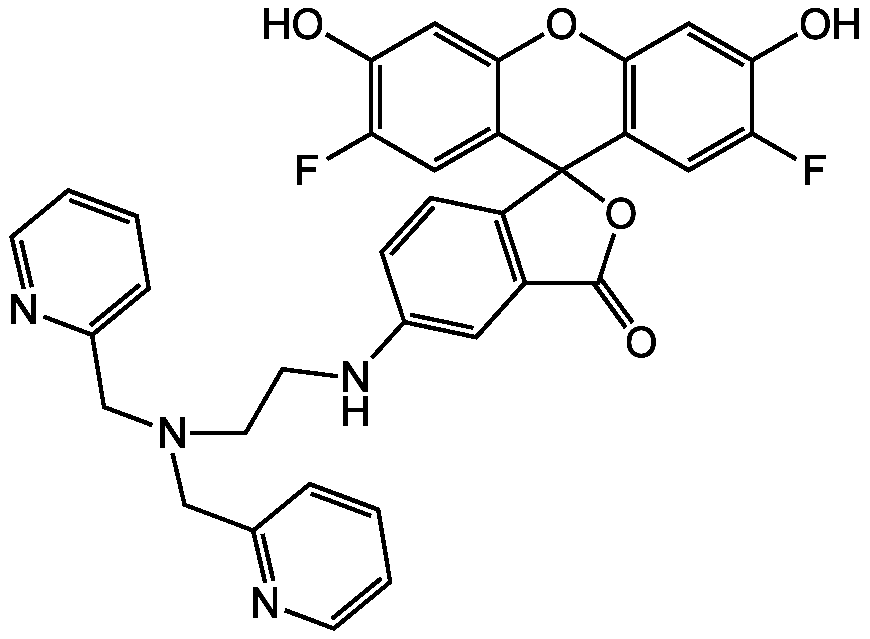
Chemical Structure
ZnAF-1F Solution [443302-08-7]
CDX-Z0509
CAS Number443302-08-7
Product group Chemicals
Estimated Purity>90%
Molecular Weight608.59
Overview
- SupplierChemodex
- Product NameZnAF-1F Solution [443302-08-7]
- Delivery Days Customer2
- CAS Number443302-08-7
- CertificationResearch Use Only
- Concentration5mM
- Estimated Purity>90%
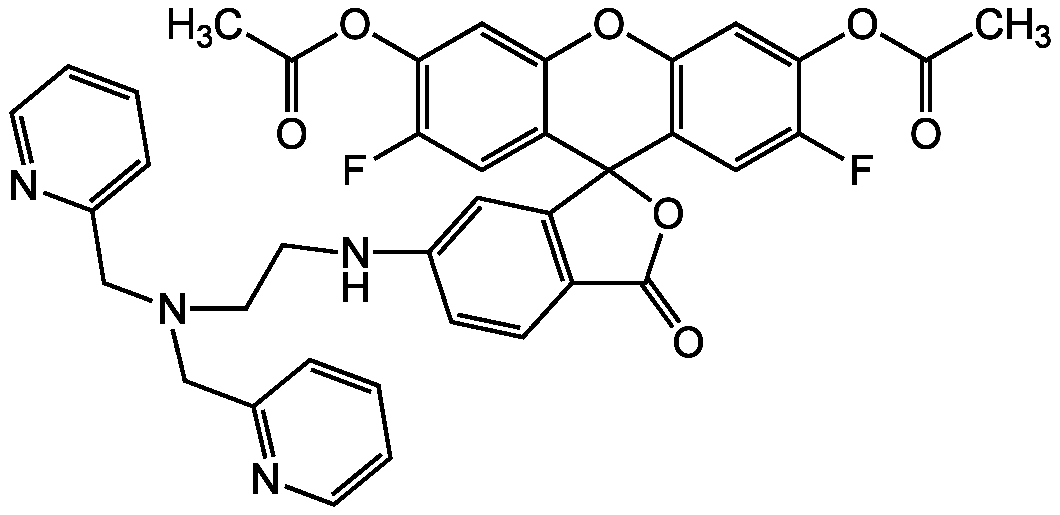
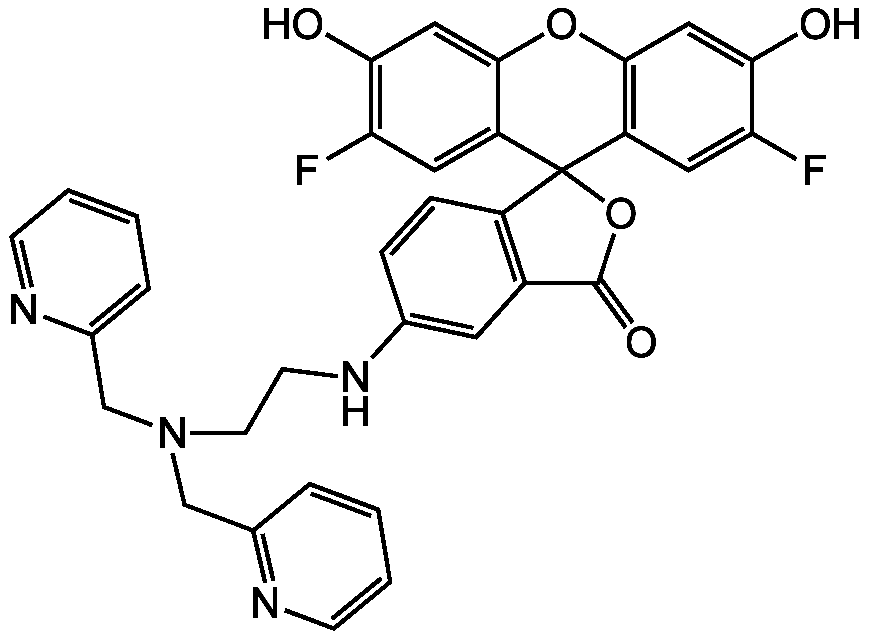
- Molecular FormulaC34H26F2N4O5
- Molecular Weight608.59
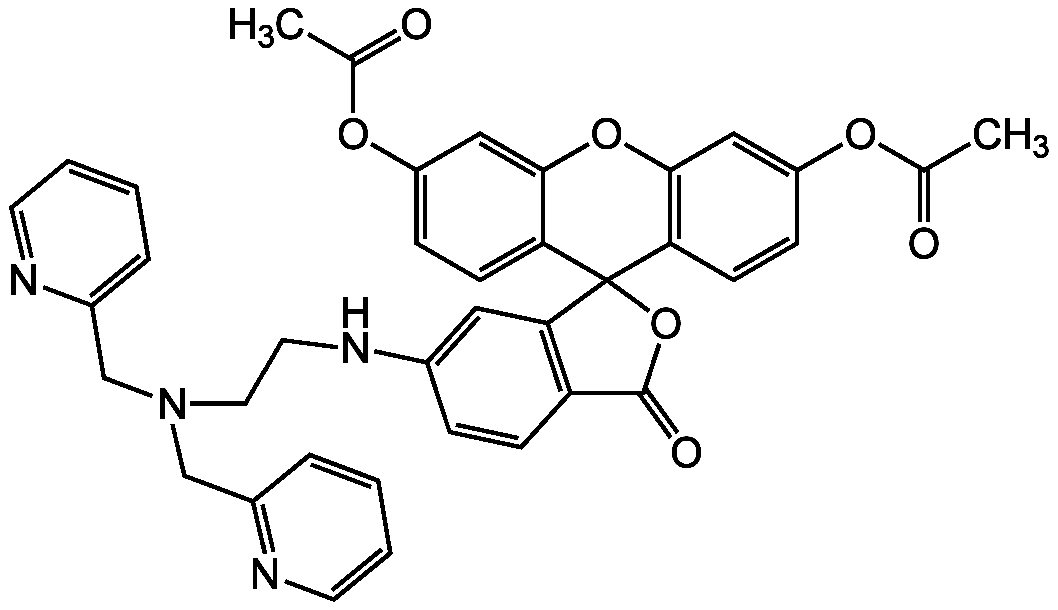
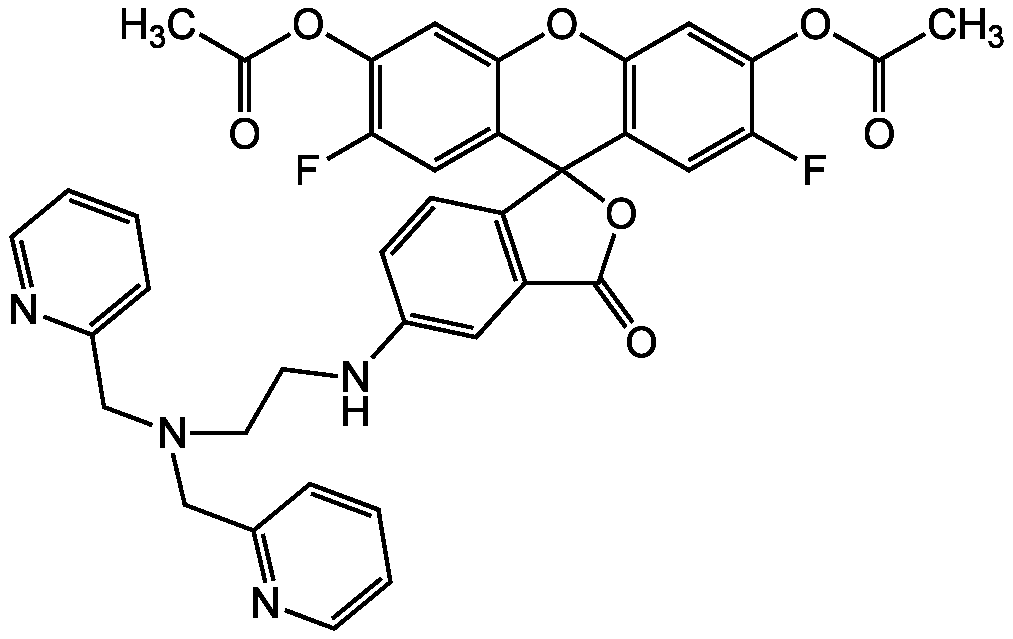
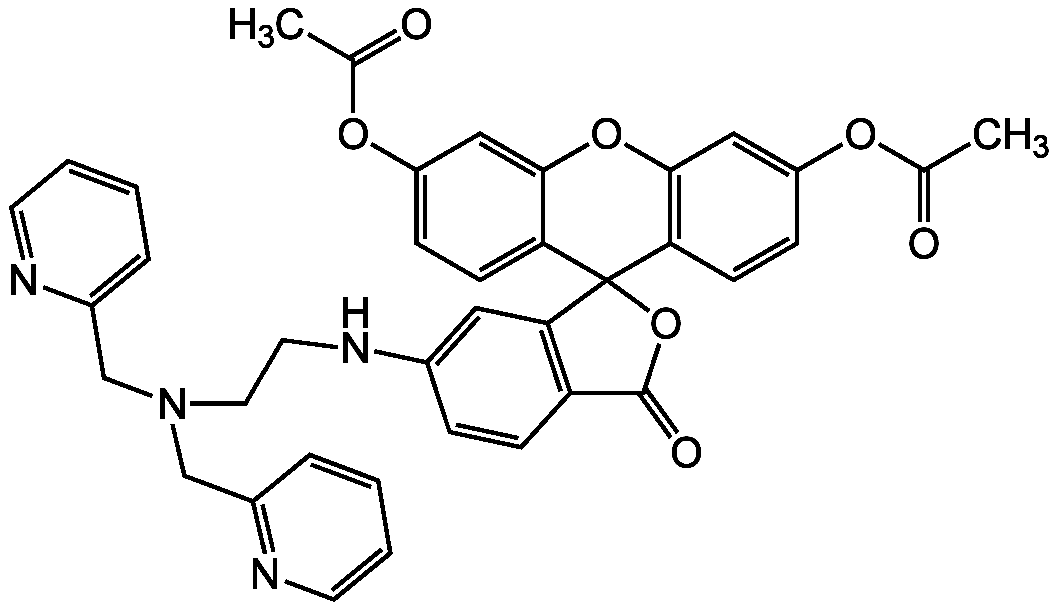
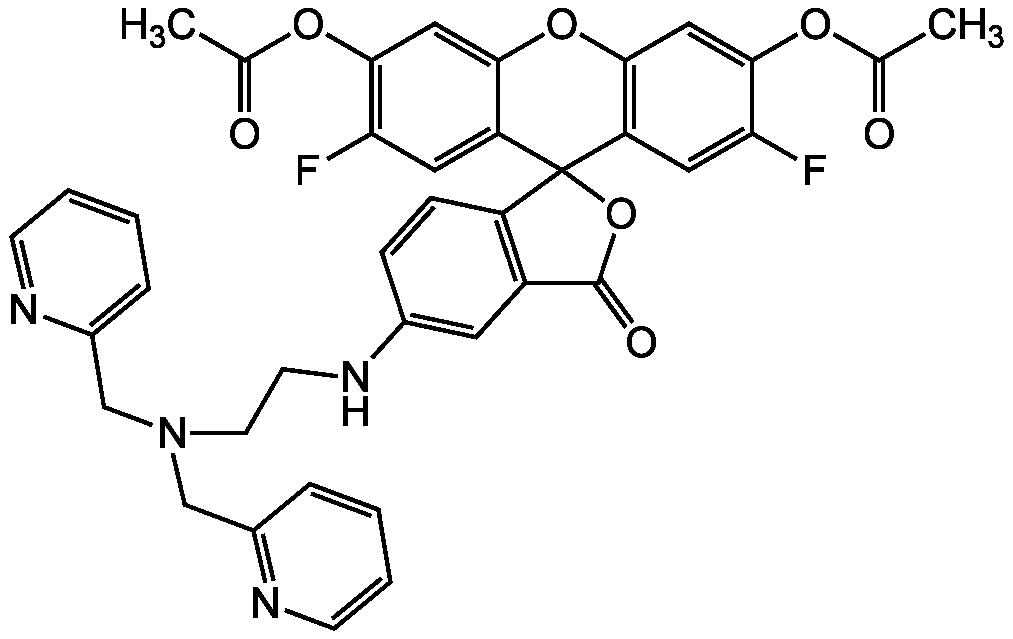
- Scientific DescriptionChemical. CAS: 443302-08-7. Formula: C34H26F2N4O5. MW: 608.59. Synthetic. ZnAF-1F and ZnAF-2F do not fluoresce in the presence of other biologically important cations such as Ca2+ and Mg2+, and are insensitive to change of pH. The complexes with Zn2+ of previously developed ZnAFs, ZnAF-1, and ZnAF-2 decrease in fluorescence intensity below pH 7.0 owing to protonation of the phenolic hydroxyl group of fluorescein, whose pKa value is 6.2. On the other hand, the Zn2+ complexes of ZnAF-1F and ZnAF-2F emit stable fluorescence under neutral and slightly acidic conditions because the pKa values are shifted to 4.9 by substitution of electron-withdrawing fluorine at the ortho position of the phenolic hydroxyl group. Spectral data: lambdaex 492nm; lambdaem 517nm in PBS. - ZnAF-1F and ZnAF-2F do not fluoresce in the presence of other biologically important cations such as Ca2+ and Mg2+, and are insensitive to change of pH. The complexes with Zn2+ of previously developed ZnAFs, ZnAF-1, and ZnAF-2 decrease in fluorescence intensity below pH 7.0 owing to protonation of the phenolic hydroxyl group of fluorescein, whose pKa value is 6.2. On the other hand, the Zn2+ complexes of ZnAF-1F and ZnAF-2F emit stable fluorescence under neutral and slightly acidic conditions because the pKa values are shifted to 4.9 by substitution of electron-withdrawing fluorine at the ortho position of the phenolic hydroxyl group. Spectral data: lambdaex 492nm; lambdaem 517nm in PBS.
- SMILESOC1=CC2=C(C=C1F)C1(OC(=O)C3=C1C=CC(NCCN(CC1=CC=CC=N1)CC1=NC=CC=C1)=C3)C1=CC(F)=C(O)C=C1O2
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12162000