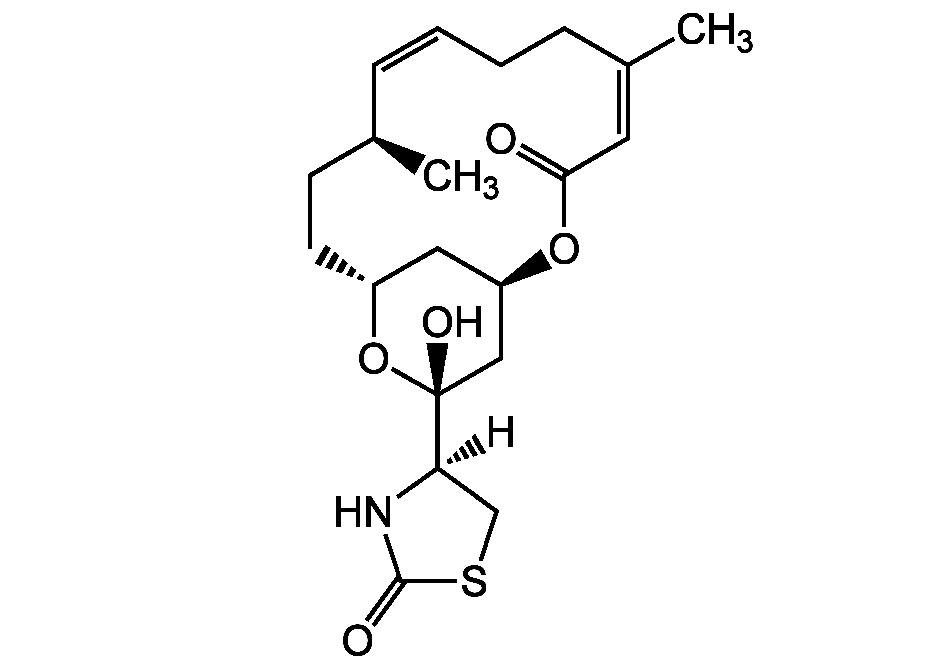
Chemical Structure
16-epi-Latrunculin B [444911-05-1] [444911-05-1]
AG-CN2-0034
CAS Number444911-05-1
Product group Chemicals
Estimated Purity>95%
Molecular Weight395.5
Overview
- SupplierAdipoGen Life Sciences
- Product Name16-epi-Latrunculin B [444911-05-1] [444911-05-1]
- Delivery Days Customer10
- CAS Number444911-05-1
- CertificationResearch Use Only
- Estimated Purity>95%
- Hazard InformationWarning
- Molecular FormulaC20H29NO5S
- Molecular Weight395.5
- Scientific DescriptionAntiviral (against herpes simplex type 1 virus (HSV-1)) [1]. Cytotoxic [1, 5]. Depolymerizes actin filaments (F-actin) [2, 3, 4]. - Chemical. CAS: 444911-05-1. Formula: C20H29NO5S. MW: 395.5. Isolated from marine sponge Negombata magnifica. Antiviral (against herpes simplex type 1 virus (HSV-1)). Cytotoxic. Depolymerizes actin filaments (F-actin).
- SMILES[H][C@@]1(CSC(=O)N1)[C@@]1(O)C[C@H]2C[C@@H](CC[C@H](C)\C=C/CC\C(C)=C/C(=O)O2)O1
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200
