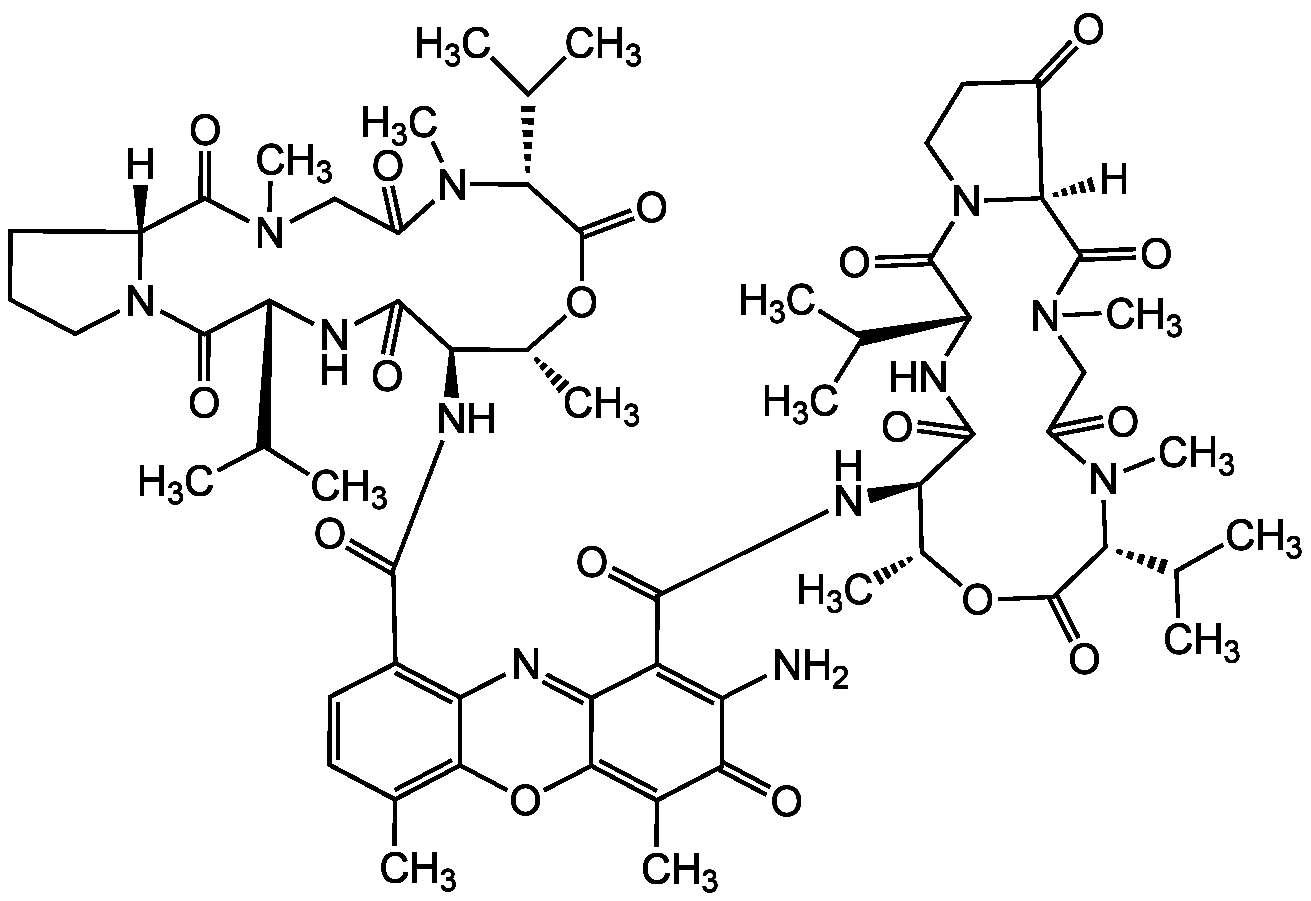
Chemical Structure
Actinomycin X2 [18865-48-0] [18865-48-0]
BVT-0375
CAS Number18865-48-0
Product group Chemicals
Estimated Purity>98%
Molecular Weight1269.4
Overview
- SupplierBioViotica
- Product NameActinomycin X2 [18865-48-0] [18865-48-0]
- Delivery Days Customer2
- CAS Number18865-48-0
- CertificationResearch Use Only
- Estimated Purity>98%
- Hazard InformationWarning
- Molecular FormulaC62H84N12O17
- Molecular Weight1269.4
- Scientific DescriptionAniso-Actinomycin (4-Oxopro instead of Pro in the beta-chain). Antitumor and antiviral antibiotic. Antituberculosis agent. Has higher cytotoxicity towards cultured human leukemia (HL-60) cells than actinomycin D. Apoptosis inducer. Forms a complex with DNA and blocks its biological activity. - Chemical. CAS: 18865-48-0. Formula: C62H84N12O17. MW: 1269.4. Isolated from Streptomyces sp. Aniso-Actinomycin (4-Oxopro instead of Pro in the beta-chain). Antitumor and antiviral antibiotic. Antituberculosis agent. Has higher cytotoxicity towards cultured human leukemia (HL-60) cells than actinomycin D. Apoptosis inducer. Forms a complex with DNA and blocks its biological activity.
- SMILES[H][C@]12CCCN1C(=O)[C@H](NC(=O)[C@H](NC(=O)C1=C3N=C4C(OC3=C(C)C=C1)=C(C)C(=O)C(N)=C4C(=O)N[C@@H]1[C@@H](C)OC(=O)[C@@H](C(C)C)N(C)C(=O)CN(C)C(=O)[C@@]3([H])N(CCC3=O)C(=O)[C@H](NC1=O)C(C)C)[C@@H](C)OC(=O)[C@@H](C(C)C)N(C)C(=O)CN(C)C2=O)C(C)C
- Storage Instruction-20°C,2°C to 8°C
- UNSPSC12352200

![Actinomycin X2 [18865-48-0] [18865-48-0]](https://www.targetmol.com/group3/M00/02/F4/CgoaEGY7QrCEGgTwAAAAAAiE5Z8268.png)